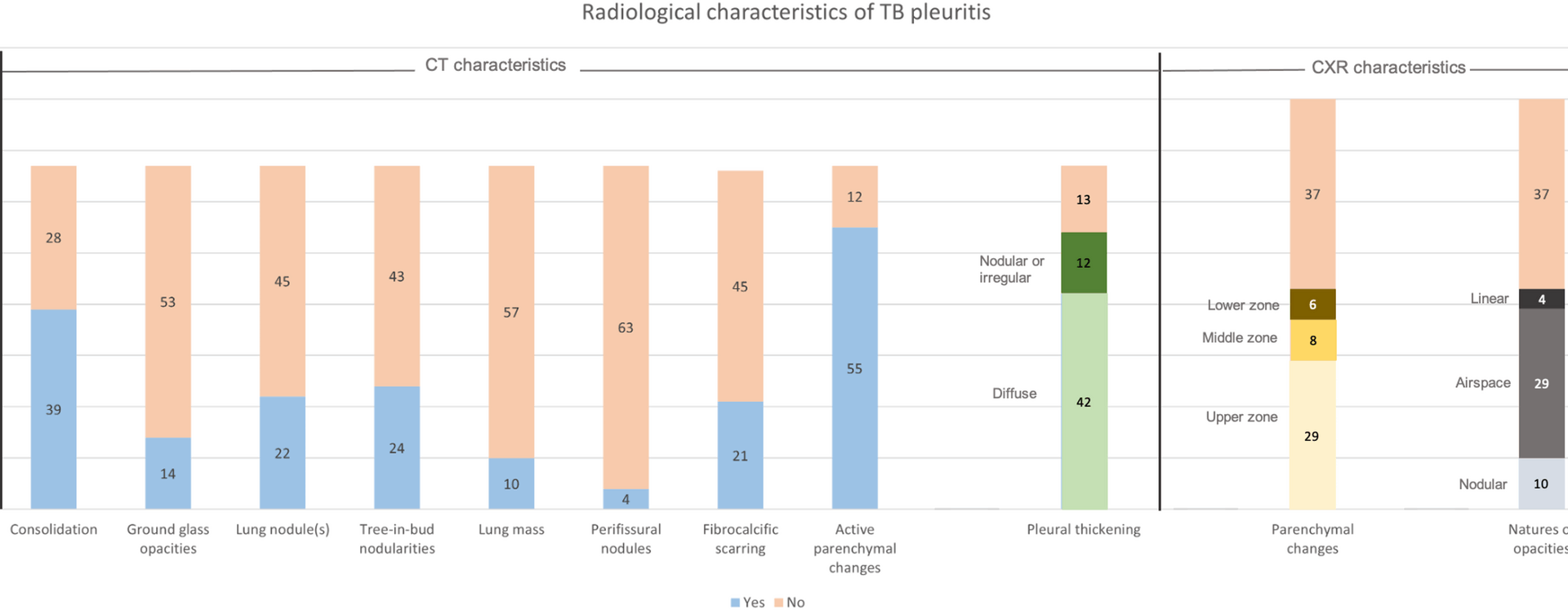
To our knowledge, few studies have described the microbiological yield of sputum and pleural investigations in relation to CT thorax findings in TB pleuritis. While the diagnosis of TB pleuritis remains challenging, the increasing accessibility to CT scans provides valuable information that can further improve the diagnostic approach to patients with suspected TB pleuritis. In this study, in 67 patients with CT imaging performed, up to 80.6% and 82.1% were found to have pleural thickening and features of active pulmonary TB on CT imaging, respectively. Furthermore, we report an association between pleural abnormalities (thickening and nodularity) on CT with a higher microbiological yield for TB pleuritis following pleural biopsy. There also appears to be a trend towards a higher sputum microbiological yield in patients with lung parenchymal abnormalities seen on CT imaging.
The prevalence of concomitant pulmonary TB in our cohort was 53.8%, based on the presence of chest radiographic parenchymal abnormalities. This is slightly higher than the majority of studies which report 17–30%[17, 20, 21] coexistent pulmonary disease based on chest radiographs. This may be because our study assessed the parenchymal changes based on the radiographs that were performed after pleural interventions, hence reducing the possibility that the effusion was obscuring the parenchymal lesions. However, we recognize that there may be a small possibility that the parenchymal changes appreciated after a pleural intervention are related to re-expansion pulmonary edema, although this is rare [22] and mitigated by controlled drainage post-intervention. Despite this, chest radiographic evidence of parenchymal disease still likely underestimates the actual prevalence of concomitant pulmonary TB, given that 82.1% of patients with CT in our study had changes suggestive of active pulmonary disease. This is similar to most existing studies reporting concomitant pulmonary TB on CT in 74–86% of cases [12, 23], and further corroborates the finding that CT is more sensitive in identifying parenchymal disease than chest radiographs in TB pleuritis [24, 25].
Importantly, there is suggestion of a higher yield of sputum microbiology in patients with CT parenchymal disease in our study. In our literature review, there was only one similar study performed by Lee et al., which was a prospective study evaluating the yield of bronchial aspirates (BA) via bronchoscopy in patients with suspected TB pleuritis [23]. They found that there was positive BA microbiology in patients with pulmonary lesions on CT in 65% of cases, compared to 7% in those without. Our findings mirror this positive correlation, even in expectorated sputum. As our study is likely underpowered to detect a statistically significant difference, larger prospective studies are needed to determine this association, which will be useful in guiding diagnostic workup in patients with suspected TB pleuritis.
Similarly, about one third (31.0%) of our patients with chest radiographic parenchymal abnormalities had positive sputum mycobacterial culture, compared to 12.5% of patients with no parenchymal changes. Although this was not a statistically significant difference, this again mirrors existing studies describing a yield of 30–33% in patients with chest radiographs demonstrating parenchymal involvement, as opposed to 9% in those without [6, 26]. The study performed by Conde et al. stands out as having a significantly higher microbiological yield of 45–55% using induced sputum, regardless of chest radiographic parenchymal disease [14]. This could be due to sputum induction being performed on all patients in the study, which has a reported yield of more than 60% in patients who are unable to expectorate sputum [27]. To the best of our knowledge, there are no studies directly comparing the yields of sputum and induced sputum. The high induced sputum microbiological yield of 55% in patients without no parenchymal disease on chest radiograph reported by Conde et al. was also inconsistent with our study as well as other existing ones. We postulate that this could be due to two reasons: Firstly, that the sensitivity of a chest radiograph may be too low to adequately identify parenchymal changes secondary to pulmonary TB, and secondly, that there were more patients with moderate or large effusions (39.0%) in the group with no parenchymal changes compared to the group that had parenchymal disease (20%) in that study. Hence, these patients could have parenchymal disease, but were not appreciated given the larger effusions.
Another key finding in our study is the positive correlation between CT pleural abnormalities and pleural tissue microbiology, but not with pleural fluid microbiology – To our knowledge, this is an interesting finding that extends from observations in prior studies. The diagnostic yield of pleural tissue (defined as either positive mycobacterial or histological result) is high, ranging from 60 to over 90% depending on procedural tool used [7, 18]. Although the demonstration of necrotizing granulomatous inflammation on pleural tissue is generally taken to be diagnostic of TB pleuritis, there are other possible pathogenic causes to consider based on patient demographics and disease endemicity [28, 29], and mycobacterial culture is key to demonstrating the offending pathogen and obtaining culture sensitivities. Our findings suggest that in patients with suspected TB pleuritis and pleural thickening or nodularity seen on imaging, microbiological diagnosis to guide anti-tuberculous treatment is best approached by obtaining pleural tissue, due to its significantly higher diagnostic yield. Pleural fluid alone does not predict microbiological yield, even in the presence of pleural disease on CT. This finding should also ideally be validated in larger cohorts, and may aid in further diagnostic algorithms in TB pleuritis.
Interestingly, we also report a high pleural fluid AFB culture yield of 50.6% in our study, compared to a yield of less than 30% which is typically reported [15]. We postulate that this may be due to a significant proportion of our patients having comorbidities of diabetes mellitus or cancer, resulting in a relative immunocompromised state due to the disease or associated treatments – this is similarly seen in HIV-infected patients with TB pleuritis [30]. Larger observational and translational models to investigate the pathophysiology of TB pleuritis may provide further insights into this.
There are limitations to our study. This was a single center retrospective study with a relatively small population size, with only half the cohort having respiratory specimens sent for microbiology and four-fifths having had CT performed. Subgroup analyses were therefore likely to be underpowered. However, the results of our study echo existing literature, and provide further insights into the various microbiological yields. The imaging findings were also not reviewed again by a dedicated thoracic radiology for the purposes of this study, but the formal radiology reports were reviewed by the investigators and incorporated into the results. We also did not conduct sputum induction or bronchoscopy for all patients, but we believe the use of expectorated sputum reflects real-life practice and limitations more accurately. This study also did not evaluate the use of thoracic ultrasound in TB pleuritis, which is increasingly being utilized to guide treatment decisions in undiagnosed pleural effusions [31, 32]. Hence, larger prospective studies are needed to validate these results and incorporate thoracic ultrasound findings to diagnostic approaches for suspected TB pleuritis.