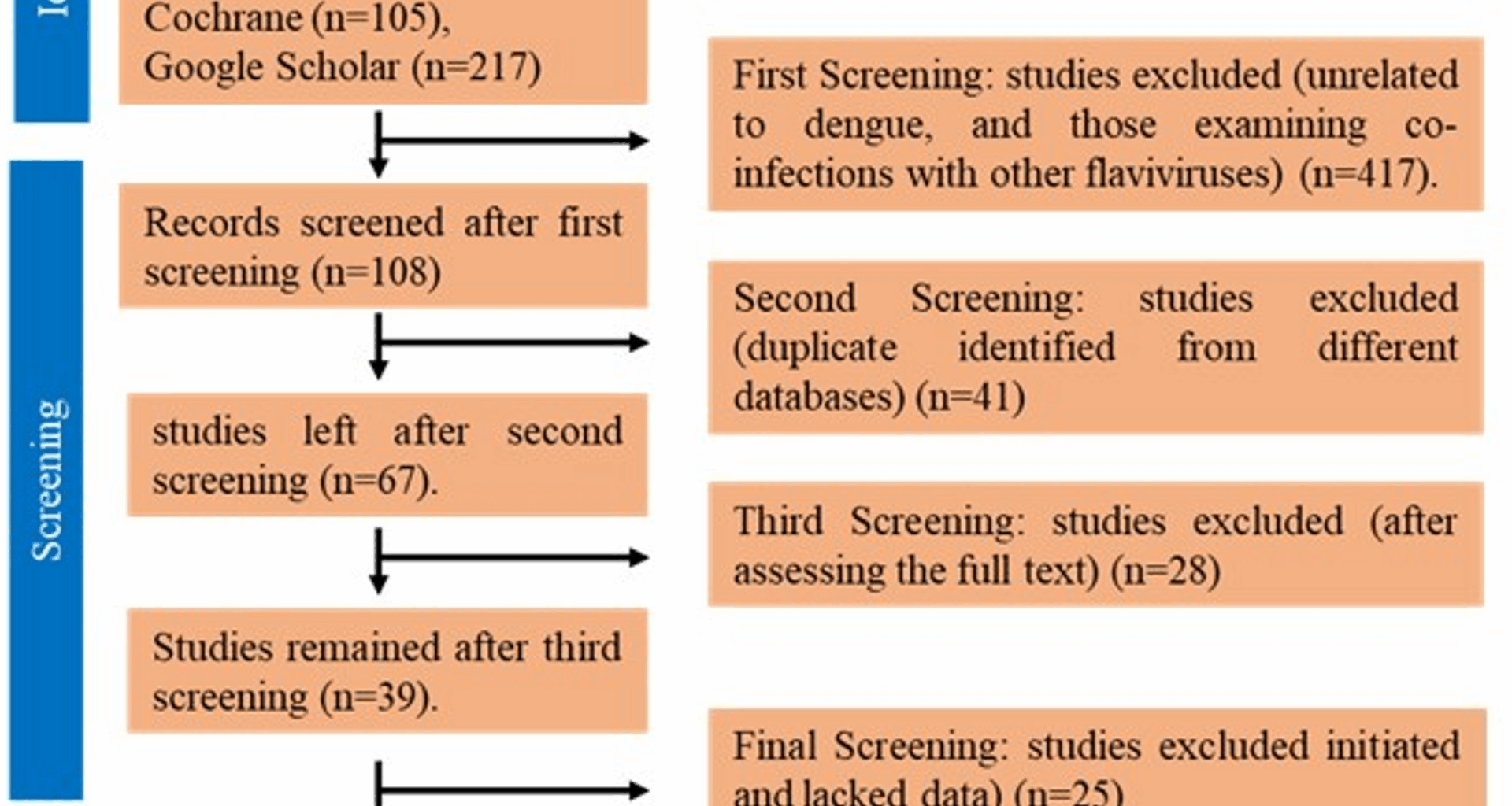
We conducted a comprehensive search for studies published in the last five years, identifying a total of 525 relevant articles. Following an initial screening, we excluded studies that were unrelated to Dengue, including focused on vaccine effects for other diseases and those examining co-infections with other flaviviruses, such as Zika and dengue. This screening reduced the selection to 108 studies. We further removed duplicate studies identified from different databases, narrowing the selection to 67 studies. Following that, a final screening was conducted, and we excluded studies that had only recently initiated and lacked data. As a result, 14 studies were found eligible for inclusion in our systematic review. Figure 1 depicts the study selection procedure. After the thorough data extraction from the screened and shortlisted studies, for a better understanding and comparison of the most efficient vaccine, all the data given in studies was incorporated into their respective tables and forest plots were made for a better presentation of the vaccine efficacy and safety report of each trial. The majority of studies focused on clinical trials of TAK-003, CYD-TDV, TV005, TV003. In addition to the aforementioned vaccines, we also identified 2 studies on the Butantan-DV and 1 study on dengue tetravalent vaccine. Even though the data from these trials is available, it was not practical to build a forest plot for these vaccines because of the small number of studies.
PRISMA flow diagram showing the selection of studies for systematic review to compare dengue vaccines safety and effectiveness
Effect measures and meta-analytical model
All statistical analyses were performed in R (v4.3.3) using the meta package (8.0.2), a comprehensive toolkit supporting fixed- and random-effects models, forest and funnel plotting, subgroup and sensitivity analyses, and bias diagnostics. Dichotomous outcomes (risk of dengue, serious adverse events) were synthesized as risk ratios (RRs) with 95% confidence intervals (CIs). We employed the DerSimonian–Laird random-effects model to accommodate between-study variation in true effect sizes, reflecting that studies differ beyond sampling error. Between-study heterogeneity was quantified using the I² statistic and Cochran’s Q test, with I² ≥ 50% indicating substantial heterogeneity. These measures help determine whether observed variability is beyond chance alone and guide interpretation of pooled estimates. We conducted pre-specified subgroup analyses by vaccine platform (TAK-003, CYD-TDV, TV005), serotype (for CYD-TDV), and age strata (< 16 vs. ≥ 16 years), using the χ² test for subgroup differences provided by the meta package. Subgroup analyses can elucidate sources of heterogeneity and explore effect modifiers. Sensitivity analyses excluded studies at high risk of bias and those with follow-up < 6 months.
TAK-003
To provide immunity to each of 4 dengue virus serotypes (DENV-1 to DENV-4), Takeda Vaccines created the live attenuated tetravalent dengue vaccine TAK-003 [9]. Engineered using a DENV-2 backbone and recombinant components of the other three serotypes, TAK-003 produces strong immunological responses mediated by humoral and T cells [10]. A pivotal five-year clinical trial involving over 20,000 children and adolescents demonstrated its efficacy in preventing severe dengue and reducing hospitalizations [11]. The European Medicines Agency approved TAK-003 in December 2022, recognizing its promise in dengue-endemic regions, followed by WHO’s Strategic Advisory Group of Experts (SAGE) endorsing its use without pre-vaccination screening in high-burden areas. TAK-003 induces neutralizing antibodies particularly effective against DENV-2 with cross-reactivity to other serotypes, enhanced by prior dengue exposure. Additionally, it stimulates memory B and T cells, contributing to sustained protection. A key benefit of TAK-003 is its capacity to reduce viremia upon subsequent dengue exposure, thereby lowering the risk of serious consequences like dengue hemorrhagic fever and dengue shock syndrome by preventing excessive inflammatory responses. While most efficacious in previously infected individuals, TAK-003 also provides substantial protection in dengue-naïve populations. Table 1 summarizes data from the clinical trials conducted over the past five years for TAK-003, while Fig. 2(a) presents a detailed analysis of its vaccine efficacy and safety profile. Pooling three RCTs (24 555 vaccinees, 12 359 controls), TAK-003 reduced risk of symptomatic or hospitalized Dengue by 85% (RR 0.15; 95% CI 0.07–0.33; I² = 95.9%). Immunogenicity analysis (1 224 vaccinees, 692 controls) showed a fourfold higher neutralizing antibody response versus placebo (RR 4.05; 95% CI 2.09–7.85; I² = 96.5%). Safety data (13 604 vaccinees, 6 819 controls) demonstrated a modest reduction in SAEs or severe solicited AEs (RR 0.85; 95% CI 0.75–0.96; I² = 0%). Subgroup testing confirmed significant differences across domains (χ² = 41.08, df = 2, p < 0.0001).
Table 1 Summary of clinical trials conducted over the past five years evaluating the efficacy and safety of TAK-003 dengue vaccineFig. 2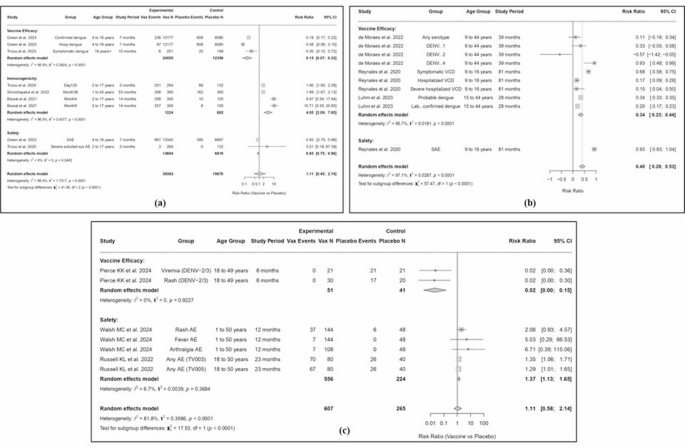
Comparison of the safety and effectiveness of dengue vaccines. The figure shows the reported frequencies of adverse effects as well as the effectiveness of dengue vaccines (a) TAK-003, (b): CYD-TDV, (c) TV003/TV005. Data is taken from randomized controlled trials and large-scale clinical studies from 2020 to 2025
CYD-TDV
Sanofi Pasteur created CYD-TDV (Dengvaxia), the first approved live attenuated tetravalent dengue vaccine, incorporating dengue virus structural genes into a yellow fever virus (YFV-17D) backbone [17]. It yields potent cellular and humoral immunological reactions, such as neutralizing antibodies and CD4+/CD8 + T cells, providing significant protection in seropositive individuals. However, in seronegative individuals, vaccine-induced antibodies may trigger ADE, heightening the chances of severe dengue [18]. Consequently, WHO recommends its use only in those with confirmed prior dengue infection. Despite these limitations, CYD-TDV is a valuable tool in endemic regions, with pre-vaccination screening essential for safe deployment. Table 2 presents a comprehensive overview of clinical trial data for CYD-TDV while Fig. 2(b) shows a detailed assessment of its efficacy and safety profile. From six RCT arms in three trials (combined N not reported here), CYD-TDV achieved a 66% reduction in any virologically confirmed dengue (RR 0.34; 95% CI 0.23–0.44; I² = 95.7%). Serotype-specific efficacy varied markedly (from non-significant for DENV-1 to 93% for DENV-4). Safety (Reynales et al.) showed fewer SAEs in vaccine versus control arms (RR 0.40; 95% CI 0.28–0.53; I² = 97.1%). Significant heterogeneity and a strong serotype effect highlight the need for serotype-targeted evaluations (χ² = 57.47, df = 1, p < 0.0001).
Table 2 Comprehensive overview of clinical trial data for the CYD-TDV dengue vaccineTV005 and TV003
Live attenuated tetravalent dengue vaccine candidates TV003 and TV005, produced by the National Institute of Allergy and Infectious Diseases (NIAID), were developed for providing adequate protection to each 4 DENV serotypes [22]. Engineered to overcome limitations of earlier vaccines, particularly the risk of ADE in seronegative individuals associated with CYD-TDV, these candidates utilize genetically modified dengue virus strains with targeted deletions to ensure safety and immunogenicity. TV005 has a greater DENV-2 component dosage, enhancing immunogenicity compared to TV003 [23]. Strong humoral and cellular immunological responses, including as neutralizing antibodies and TC and TH activation, are elicited by both vaccinations. TNF-α and IFN-γ induction further enhances long-term memory and strong cellular immunity. Crucially, TV003 and TV005 demonstrate efficacy in both seropositive and seronegative individuals without requiring pre-vaccination screening. Their single-dose regimen and robust, durable protection position them as highly promising candidates for large-scale dengue prevention efforts globally. Table 3 summarizes data from the clinical trials conducted over the past five years for TV005 and TV003, while Fig. 2(c) presents a detailed analysis of its vaccine efficacy and safety profile. Two challenge-based RCTs (51 vaccinees, 41 controls) demonstrated near‐complete protection against DENV-2/3 viremia and rash (pooled RR 0.02; 95% CI 0.00–0.15; I² = 0%). Reactogenicity data (n = 556 vaccinees vs. 224 controls) indicated an increased risk of any solicited AE (RR 1.37; 95% CI 1.13–1.65; I² = 6.7%), driven by rash, fever, and arthralgia. Subgroup differences between efficacy and safety were significant (χ² = 17.53, df = 1, p < 0.0001).
Table 3 Summary of clinical trials conducted over the past five years evaluating the efficacy and safety of TV005 and TV003 dengue vaccineOther vaccine trials
Studies on the Butantan-DV and Dengue tetravalent vaccine were also identified alongside the previously mentioned ones. Butantan DV vaccine, developed by the Brazilian Butantan Institute, is a live attenuated tetravalent dengue vaccine designed to offer balanced, strong immunity for each of the 4 DENV serotypes [26]. Based on the TV003/TV005 formulations, it has been optimized for safety and efficacy in dengue-endemic regions. Unlike CYD-TDV (Dengvaxia), which poses a risk of antibody-dependent enhancement (ADE) in seronegative individuals, Butantan DV provides protection for both seropositive and seronegative individuals. The vaccine stimulates robust humoral and cellular immunity, producing neutralizing antibodies against the envelope (E) and precursor membrane (prM) proteins and activating CD4 + and CD8 + T cells. These responses reduce viral replication, prevent severe outcomes like dengue hemorrhagic fever (DHF) and shock syndrome (DSS), and promote long-term immune memory. A key advantage is its prospective single-dose regimen, simplifying mass immunization efforts. Genetically engineered with reduced replication capacity, Butantan DV maintains high immunogenicity while ensuring safety, making it a promising tool for global Dengue control. Conversely, Panacea Biotec Ltd has created DengiAll, a tetravalent dengue vaccine. Immunity compared to all 4 Dengue virus serotypes is intended to be provided by this live-attenuated, lyophilized vaccine that is generated from cell cultures. The vaccine is presently in Phase 3 of clinical testing, which started in 2019. The data analysis of Butantan DV and DengiAll is shown in Table 4.
Table 4 Comprehensive overview of clinical trial data for the Butantan DV and DengiAll