Sperm cells are among the most specialized in the body, designed for a single purpose: fertilization. Each carries DNA and propels itself toward an egg using a whip-like tail called a flagellum. Inside the testes, sperm develop through several well-defined stages, acquiring the structures and molecules needed for fertilization.
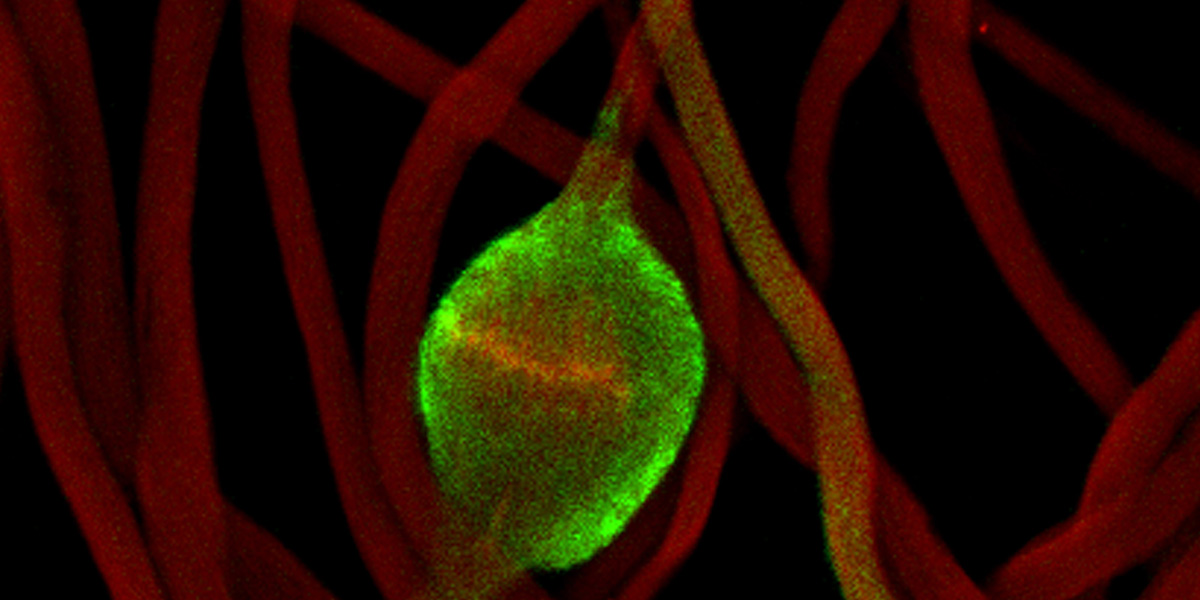
Hermann Steller, Rockefeller University, via National Institutes of Health, National Institute of General Medical Sciences image gallery.
Developing fruit fly spermatids require caspase activity (green) for the elimination of unwanted organelles and cytoplasm via apoptosis.
One crucial part of this transformation is the production of seminolipids, specialized fats found in developing sperm that are essential for their formation and function. Seminolipids are synthesized by fatty acyl-CoA reductase, or FAR, enzymes. Mammals have two different FAR enzymes, FAR1 and FAR2.
Although both FAR1 and FAR2 are known to synthesize fatty alcohols, their specific roles in seminolipid production had remained unclear. To pinpoint which enzyme was responsible, Ayano Tamazawa and colleagues at Hokkaido University analyzed mice lacking Far1 and Far2 to clarify their roles in seminolipid production and spermatogenesis. They found that loss of Far1 led to a dramatic decrease in seminolipids and impaired sperm development. The study was published in the Journal of Biological Chemistry.
Tamazawa said the findings show how the loss of seminolipids disrupts spermatogenesis, emphasizing the critical role of ether linkages in sperm development.
Seminolipids are categorized by the types of alkyl and acyl chains they contain, which differ in length and saturation. The most common seminolipid in the testis is O-C16:0/C16:0. Using liquid chromatography–tandem mass spectrometry, or LC–MS/MS, the researchers mapped the exact structure of these lipids.
High-resolution lipidomics analysis of seminolipids and SGalDAGs (3-sulfogalactosyl-1-acyl-2-acylglycerols) showed that both lipid types have similar side chains composed of saturated acyl or alkyl groups. The main difference is that SGalDAGs have a 1-acyl group, whereas seminolipids have a 1-alkyl group.
“This finding is unique because SGalDAGs have never been characterized in detail in the testis,” Tamazawa said.
However, the precise mechanisms by which seminolipids contribute to spermatogenesis, and why the C16:0/C16:0 structure predominates, remain unknown.
The researchers suggest that understanding seminolipid function could inform new diagnostic or therapeutic strategies for male infertility, potentially leading to lipid supplements or biomarkers.