World Health Organization. Regulatory Considerations on Artificial Intelligence. https://iris.who.int/bitstream/handle/10665/373421/9789240078871-eng.pdf?sequence=1&isAllowed=y (2023).
Widder, D. G., Whittaker, M. & West, S. M. Why ‘open’ AI systems are actually closed, and why this matters. Nature 635, 827–833 (2024).
Lavigne, M., Mussa, F., Creatore, M. I., Hoffman, S. J. & Buckeridge, D. L. A population health perspective on artificial intelligence. Health. Manag. Forum 32, 173–177 (2019).
World Health Organization. Ethics and Governance of Artificial Intelligence for Health: Guidance on Large Multi-Modal Models. https://www.who.int/publications/i/item/9789240084759 (2024).
Sampson, C. et al. Digital cognitive behavioural therapy for insomnia and primary care costs in England: an interrupted time series analysis. BJGP Open 6, BJGPO.2021.0146 (2022).
Areia, M. et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digital Health 4, e436–e444 (2022).
Kumar, S., Jones Bell, M. & Juusola, J. L. Mobile and traditional cognitive behavioral therapy programs for generalized anxiety disorder: A cost-effectiveness analysis. PLoS ONE 13, e0190554 (2018).
van Kessel, R. et al. Mapping Factors That Affect the Uptake of Digital Therapeutics Within Health Systems: Scoping Review. J. Med. Internet Res. 25, e48000 (2023).
Reddy, S. et al. Evaluation framework to guide implementation of AI systems into healthcare settings. BMJ Health Care Inf. 28, e100444 (2021).
Han, R. et al. Randomised controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digital Health 6, e367–e373 (2024).
Schmit, C. D., Doerr, M. J. & Wagner, J. K. Leveraging IP for AI governance. Science 379, 646–648 (2023).
Picht, P. G. & Thouvenin, F. AI and IP: Theory to Policy and Back Again – Policy and Research Recommendations at the Intersection of Artificial Intelligence and Intellectual Property. IIC 54, 916–940 (2023).
Schmidt, J. et al. Mapping the regulatory landscape for artificial intelligence in health within the European Union. npj Digital Med. 7, 229 (2024).
Gilmore, A. B. et al. Defining and conceptualising the commercial determinants of health. Lancet 401, 1194–1213 (2023).
Duffourc, M. & Gerke, S. Generative AI in Health Care and Liability Risks for Physicians and Safety Concerns for Patients. JAMA 330, 313–314 (2023).
Navigli, R., Conia, S. & Ross, B. Biases in Large Language Models: Origins, Inventory, and Discussion. J. Data Info. Qual. 15, 10:1–10:21 (2023).
van Kessel, R. et al. A scoping review and expert consensus on digital determinants of health. Bull. World Health Organ 103, 110–125H (2025).
The Lancet Global Health. Advancing equity in medical device performance. Lancet Glob. Health 12, e712 (2024).
Ibrahim, H., Liu, X., Zariffa, N., Morris, A. D. & Denniston, A. K. Health data poverty: an assailable barrier to equitable digital health care. Lancet Digital Health 3, e260–e265 (2021).
Floridi, L. & Cowls, J. A Unified Framework of Five Principles for AI in Society. Harvard Data Sci. Rev. 1, 1–15 (2019).
Shin, D. & Park, Y. J. Role of fairness, accountability, and transparency in algorithmic affordance. Computers Hum. Behav. 98, 277–284 (2019).
Solaiman, B. & Malik, A. Regulating algorithmic care in the European Union: evolving doctor–patient models through the Artificial Intelligence Act (AI-Act) and the liability directives. Medical Law Review fwae033 https://doi.org/10.1093/medlaw/fwae033 (2024).
Serra-Burriel, M., Locher, L. & Vokinger, K. N. Development Pipeline and Geographic Representation of Trials for Artificial Intelligence/Machine Learning–Enabled Medical Devices (2010 to 2023). NEJM AI 1, AIp2300038 (2023).
Muehlematter, U. J., Daniore, P. & Vokinger, K. N. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): a comparative analysis. Lancet Digital Health 3, e195–e203 (2021).
World Health Organization. Leading the Realization of Human Rights to Health and through Health: Report of the High-Level Working Group on the Health and Human Rights of Women, Children and Adolescents. https://iris.who.int/handle/10665/255540 (2017).
van Kessel, R. et al. Autism and education—international policy in small EU states: policy mapping in Malta, Cyprus, Luxembourg and Slovenia. Eur. J. Public Health 30, 1078–1083 (2020).
van Kessel, R. et al. Autism, Austerity, and the Right to Education in the EU: Policy Mapping and Scoping Review of Ireland, Portugal, Italy, and Greece. Eur. Policy Anal. 00, 1–13 (2021).
United Nations General Assembly. International Covenant on Economic, Social and Cultural Rights. (1966).
World Intellectual Property Organization. Berne Convention for the Protection of Literary and Artistic Works (as Amended on September 28, 1979). https://www.wipo.int/wipolex/en/text/283698 (1979).
World Intellectual Property Organization. WIPO Copyright Treaty. https://www.wipo.int/wipolex/en/text/295166 (1996).
World Trade Organization. Agreement on Trade-Related Aspects of Intellectual Property Rights (as Amended on 23 January 2017). https://www.wto.org/english/docs_e/legal_e/downloads_e/TRIPS05_en.pdf (2005).
European Commission. Directive 96/9/EC of the European Parliament and of the Council of 11 March 1996 on the Legal Protection of Databases. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01996L0009-20190606 (1996).
European Commission. Regulation (EU) 2023/2854 of the European Parliament and of the Council of 13 December 2023 on Harmonised Rules on Fair Access to and Use of Data and Amending Regulation (EU) 2017/2394 and Directive (EU) 2020/1828 (Data Act). (2023).
European Commission. Regulation (EU) 2022/1925 of the European Parliament and of the Council of 14 September 2022 on Contestable and Fair Markets in the Digital Sector and Amending Directives (EU) 2019/1937 and (EU) 2020/1828 (Digital Markets Act) (Text with EEA Relevance). https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32022R1925 (2022).
European Commission. General Data Protection Regulation. https://eur-lex.europa.eu/eli/reg/2016/679/oj (2016).
European Commission. Regulation (EU) 2025/327 of the European Parliament and of the Council of 11 February 2025 on the European Health Data Space and Amending Directive 2011/24/EU and Regulation (EU) 2024/2847 (Text with EEA Relevance). https://eur-lex.europa.eu/eli/reg/2025/327/oj/eng (2025).
European Commission. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC (Text with EEA Relevance)Text with EEA Relevance. https://eur-lex.europa.eu/eli/reg/2014/536/2022-12-05 (2014).
European Commission. Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/EU. https://eur-lex.europa.eu/eli/reg/2017/746/oj/eng (2017).
European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA Relevance). OJ L vol. 117 (2017).
European Commission. Regulation (EU) 2024/1689 of the European Parliament and of the Council of 13 June 2024 Laying down Harmonised Rules on Artificial Intelligence and Amending Regulations (EC) No 300/2008, (EU) No 167/2013, (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1139 and (EU) 2019/2144 and Directives 2014/90/EU, (EU) 2016/797 and (EU) 2020/1828 (Artificial Intelligence Act). https://eur-lex.europa.eu/eli/reg/2024/1689/oj (2024).
European Commission. Directive (EU) 2016/943 of the European Parliament and of the Council of 8 June 2016 on the Protection of Undisclosed Know-How and Business Information (Trade Secrets) against Their Unlawful Acquisition, Use and Disclosure. https://eur-lex.europa.eu/eli/dir/2016/943/oj/eng (2016).
Porxas, N. & Sanz, C. AI Health Applications and Related Intellectual Property Challenges. EPLR 3, 184–191 (2019).
European Patent Office. European Patent Convention. https://www.epo.org/en/legal/epc/2020/index.html (2020).
World Trade Organization. Doha Declaration on the TRIPS Agreement and Public Health. https://www.wto.org/English/thewto_e/minist_e/min01_e/mindecl_trips_e.htm (2001).
European Commission. Directive 2001/29/EC of the European Parliament and of the Council of 22 May 2001 on the Harmonisation of Certain Aspects of Copyright and Related Rights in the Information Society. https://eur-lex.europa.eu/eli/dir/2001/29/oj/eng (2001).
Hohfeld, W. N. Fundamental Legal Conceptions as Applied in Judicial Reasoning. Yale Law J. 26, 710–770 (1917).
Council of Europe. European Convention on Human Rights. https://www.echr.coe.int/documents/d/echr/Convention_ENG (1950).
Council of Europe. Framework Convention on Artificial Intelligence and Human Rights, Democracy and the Rule of Law. https://rm.coe.int/1680afae3c (2024).
United Nations General Assembly. International Covenant on Civil and Political Rights. (1966).
Aboy, M., Lath, A., Minssen, T. & Liddell, K. The sufficiency of disclosure of AI inventions. J. Intellect. Prop. Law Pract. 19, 834–840 (2024).
Aplin, T. The limits of trade secret protection in the EU. in Research Handbook on Information Law and Governance 174–194 (Edward Elgar Publishing, 2021).
Ungureanu, C. T. & Tataru, ÇR. The legality of reverse engineering or how to legally decipher trade secrets. SHS Web Conf. 177, 02001 (2023).
Pasquale, F. The Black Box Society: The Secret Algorithms That Control Money and Information. Harvard University Press: Cambridge, Massachusetts London, England, 2016.
Durán, J. M. & Jongsma, K. R. Who is afraid of black box algorithms? On the epistemological and ethical basis of trust in medical AI. J. Med Ethics 47, 329–335 (2021).
van Kolfschooten, H. B. A health-conformant reading of the GDPR’s right not to be subject to automated decision-making. Med. Law Rev. 32, 373–391 (2024).
Minssen, T., Mimler, M. & Mak, V. When Does Stand-Alone Software Qualify as a Medical Device in the European Union?—The Cjeu’s Decision in Snitem and What it Implies for the Next Generation of Medical Devices. Med. Law Rev. 28, 615–624 (2020).
van Kolfschooten, H. The AI cycle of health inequity and digital ageism: mitigating biases through the EU regulatory framework on medical devices. J. Law Biosci. 10, lsad031 (2023).
Kulkov, I. Next-generation business models for artificial intelligence start-ups in the healthcare industry. Int. J. Entrepreneurial Behav. Res. 29, 860–885 (2021).
Sekalala, S. et al. Decolonising human rights: how intellectual property laws result in unequal access to the COVID-19 vaccine. BMJ Glob. Health 6, e006169 (2021).
Burk, D. L. Patents as Data Aggregators in Personalized Medicine. SSRN Scholarly Paper at https://papers.ssrn.com/abstract=2597525 (2015).
Khan, S. & Mian, A. Racism and medical education. Lancet Infect. Dis. 20, 1009 (2020).
Amutah, C. et al. Misrepresenting Race — The Role of Medical Schools in Propagating Physician Bias. N. Engl. J. Med. 384, 872–878 (2021).
Norori, N., Hu, Q., Aellen, F. M., Faraci, F. D. & Tzovara, A. Addressing bias in big data and AI for health care: A call for open science. Patterns (N. Y) 2, 100347 (2021).
European Patent Office. J 0008/20 (Designation of Inventor/DABUS) 21-12-2021. https://www.epo.org/en/boards-of-appeal/decisions/j200008eu1 (2021).
United Kingdom Supreme Court. Thaler (Appellant) v Comptroller-General of Patents, Designs and Trademarks (Respondent). https://supremecourt.uk/uploads/uksc_2021_0201_judgment_3f445a5dc7.pdf (2023).
Saravanan, A. & Deva Prasad, M. AI as an Inventor Debate under the Patent Law: A Post-DABUS Comparative Analysis. SSRN Scholarly Paper at https://doi.org/10.2139/ssrn.5053108 (2024).
Arora, A., Wagner, S. K., Carpenter, R., Jena, R. & Keane, P. A. The urgent need to accelerate synthetic data privacy frameworks for medical research. Lancet Digit. Health https://doi.org/10.1016/S2589-7500(24)00196-1 (2024).
Longpre, S. et al. A large-scale audit of dataset licensing and attribution in AI. Nat. Mach. Intell. 6, 975–987 (2024).
Amann, J. et al. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inf. Decis. Mak. 20, 310 (2020).
LaRosa, E. & Danks, D. Impacts on Trust of Healthcare AI. in Proceedings of the 2018 AAAI/ACM Conference on AI, Ethics, and Society 210–215 (ACM, New Orleans LA USA, 2018). https://doi.org/10.1145/3278721.3278771.
Murphy, K. et al. Artificial intelligence for good health: a scoping review of the ethics literature. BMC Med Ethics 22, 14 (2021).
Kreps, S., George, J., Lushenko, P. & Rao, A. Exploring the artificial intelligence “Trust paradox”: Evidence from a survey experiment in the United States. PLoS One 18, e0288109 (2023).
Independent High-level Expert Group on Artificial Intelligence. Ethics Guidelines for Trustworthy AI. https://digital-strategy.ec.europa.eu/en/library/ethics-guidelines-trustworthy-ai (2019).
Kiseleva, A., Kotzinos, D. & De Hert, P. Transparency of AI in Healthcare as a Multilayered System of Accountabilities: Between Legal Requirements and Technical Limitations. Front. Artificial. Intell. 5, 879603 (2022).
Karpathakis, K., Morley, J. & Floridi, L. A Justifiable Investment in AI for Healthcare: Aligning Ambition with Reality. Minds Mach. 34, 38 (2024).
Kostick-Quenet, K., Lang, B. H., Smith, J., Hurley, M. & Blumenthal-Barby, J. Trust criteria for artificial intelligence in health: normative and epistemic considerations. J. Med. Ethics 50, 544–551 (2024).
Hua, D., Petrina, N., Young, N., Cho, J.-G. & Poon, S. K. Understanding the factors influencing acceptability of AI in medical imaging domains among healthcare professionals: A scoping review. Artif. Intell. Med. 147, 102698 (2024).
Barnett, J. AI Ecosystem Poses IP Risk and Antitrust Considerations. Bloomberg Law (2024).
Rosati, E. Is text and data mining synonymous with AI training?. J. Intellect. Prop. Law Pract. 19, 851–852 (2024).
Dornis, T. W. The Training of Generative AI Is Not Text and Data Mining. SSRN Scholarly Paper at https://doi.org/10.2139/ssrn.4993782 (2024).
Schirru, L., Rocha de Souza, A., Valente, M. G. & de Perdigão Lana, A. Text and Data Mining Exceptions in Latin America. IIC 55, 1624–1653 (2024).
Tyagi, K. Copyright, text & data mining and the innovation dimension of generative AI. J. Intellect. Prop. Law Pract. 19, 557–570 (2024).
Wintemute, R. The European Convention on Human Rights: Immutable Status and Sex Discrimination Arguments. in Sexual Orientation and Human Rights (ed. Wintemute, R.) 0 (Oxford University Press, 1993). https://doi.org/10.1093/acprof:oso/9780198264880.003.0005.
Bryant, M. Denmark to tackle deepfakes by giving people copyright to their own features. The Guardian (2025).
van Kessel, R., Czabanowska, K. & Roman-Urrestarazu, A. Systematically mapping and analysing multi-level policy developments: a methodological toolkit. Preprint at https://doi.org/10.21203/rs.3.rs-3788502/v1 (2023).
Arksey, H. & O’Malley, L. Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 8, 19–32 (2005).
Levac, D., Colquhoun, H. & O’Brien, K. K. Scoping studies: advancing the methodology. Implement. Sci. 5, 69 (2010).
van Kessel, R. et al. Education, Special Needs, and Autism in the Baltic States: Policy Mapping in Estonia, Latvia, and Lithuania. Front. Educ. 5, 161 (2020).
Bunt, D. et al. Quotas, and Anti-discrimination Policies Relating to Autism in the EU: Scoping Review and Policy Mapping in Germany, France, Netherlands, United Kingdom, Slovakia, Poland, and Romania. Autism Res. 13, 1397–1417 (2020).
van Kessel, R. et al. Digital Health Reimbursement Strategies of 8 European Countries and Israel: Scoping Review and Policy Mapping. JMIR mHealth uHealth 11, e49003 (2023).
Neicun, J. et al. Mapping novel psychoactive substances policy in the EU: The case of Portugal, the Netherlands, Czech Republic, Poland, the United Kingdom and Sweden. PLOS ONE 14, e0218011 (2019).
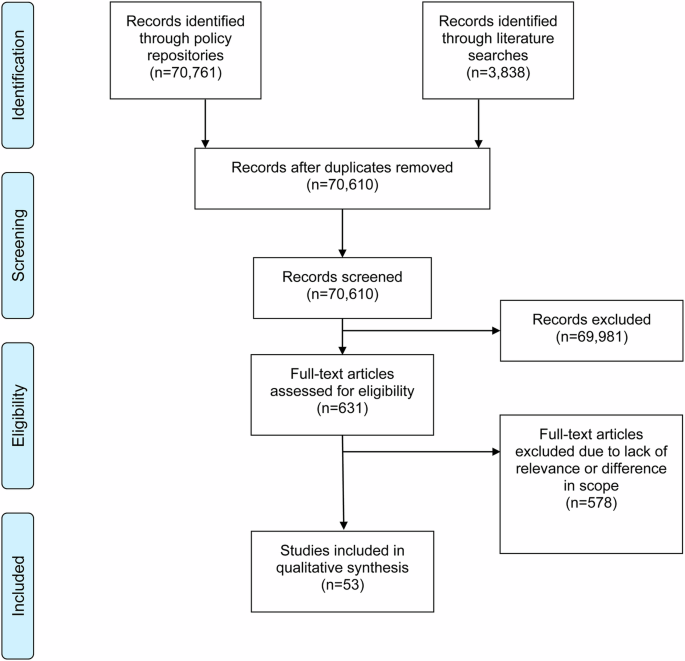
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, T. P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 6, e1000097 (2009).
Tricco, A. C. et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern Med 169, 467–473 (2018).
Dalglish, S. L., Khalid, H. & McMahon, S. A. Document analysis in health policy research: the READ approach. Health Policy Plan. 35, 1424–1431 (2020).
Marshall, M. N. Sampling for qualitative research. Fam. Pract. 13, 522–525 (1996).
Eurostat. Population on 1 January. https://ec.europa.eu/eurostat/databrowser/view/tps00001/default/table?lang=en&category=t_demo.t_demo_pop (2023).
van Kessel, R., Wong, B. L. H., Rubinić, I., O’Nuallain, E. & Czabanowska, K. Is Europe prepared to go digital? making the case for developing digital capacity: An exploratory analysis of Eurostat survey data. PLOS Digital Health 1, e0000013 (2022).
Eurostat. Use of artificial intelligence in enterprises. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Use_of_artificial_intelligence_in_enterprises (2024).
European Commission. Treaty on the Functioning of the European Union. OJ C vol. 326 (2009).
Margoni, T. The Harmonisation of EU Copyright Law: The Originality Standard. in Global Governance of Intellectual Property in the 21st Century: Reflecting Policy Through Change (ed. Perry, M.) 85–105 (Springer International Publishing, Cham, 2016). https://doi.org/10.1007/978-3-319-31177-7_6.
World Intellectual Property Organization. Paris Convention for the Protection of Industrial Property. https://www.wipo.int/wipolex/en/text/288514 (1979).
Haddaway, N. R., Collins, A. M., Coughlin, D. & Kirk, S. The Role of Google Scholar in Evidence Reviews and Its Applicability to Grey Literature Searching. PLOS ONE 10, e0138237 (2015).
Braun, V. & Clarke, V. Using thematic analysis in psychology. Qualitative Res. Psychol. 3, 77–101 (2006).
Kyngäs, H. Inductive Content Analysis. in The Application of Content Analysis in Nursing Science Research (eds Kyngäs, H., Mikkonen, K. & Kääriäinen, M.) 13–21 (Springer International Publishing, Cham, 2020). https://doi.org/10.1007/978-3-030-30199-6_2.