Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med. 2021;385:1018–32.
Criado E, Sanchez M, Ramirez J, Arguis P, de Caralt TM, Perea RJ, Xaubet A. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30:1567–86.
Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, Abston E, Bernstein RC, Blankstein R, Chen ES. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26–51.
Seve P, Pacheco Y, Durupt F, Jamilloux Y, Gerfaud-Valentin M, Isaac S, Boussel L, Calender A, Androdias G, Valeyre D. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766.
Rossides M, Darlington P, Kullberg S, Arkema EV. Sarcoidosis: epidemiology and clinical insights. J Intern Med. 2023;293:668–80.
Gerke AK. Treatment of sarcoidosis: a multidisciplinary approach. Front Immunol. 2020;11:545413.
Guleria R, Mahashur A, Ghoshal AG, Thomas PK, Raghu G, Baughman RP. Challenges in diagnosing sarcoidosis in tuberculosis endemic regions: clinical scenario in India. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:381–4.
Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Sarcoidosis and tuberculosis: the same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18:506–16.
Patterson KC, Queval CJ, Gutierrez MG. Granulomatous inflammation in tuberculosis and sarcoidosis: does the lymphatic system contribute to disease? BioEssays. 2019;41:1900086.
Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics. 2004;24:87–104.
Andreu J, Caceres J, Pallisa E, Martinez-Rodriguez M. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol. 2004;51:139–49.
Saidha S, Sotirchos ES, Eckstein C. Etiology of sarcoidosis: does infection play a role? Yale J Biol Med. 2012;85:133.
Pagán AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med. 2015;5:a018499.
Zhou Y, Li HP, Li QH, Zheng H, Zhang RX, Chen G, Baughman RP. Differentiation of sarcoidosis from tuberculosis using real-time PCR assay for the detection and quantification of Mycobacterium tuberculosis. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:93–9.
Talwar H, Rosati R, Li J, Kissner D, Ghosh S, Fernandez-Madrid F, Samavati L. Development of a T7 phage display library to detect sarcoidosis and tuberculosis by a panel of novel antigens. EBioMedicine. 2015;2:341–50.
Costabel U, Bonella F, Ohshimo S, Guzman J. Diagnostic modalities in sarcoidosis: BAL, EBUS, and PET. Semin Respir Crit Care Med. 2010;31:404–8.
Gilpin C, Korobitsyn A, Migliori GB, Raviglione MC, Weyer K. The World Health Organization standards for tuberculosis care and management. 2018.
World Health Organization. Early detection of tuberculosis: an overview of approaches, guidelines and tools. 2011.
MacNeil A, Glaziou P, Sismanidis C, Date A, Maloney S, Floyd K. Global epidemiology of tuberculosis and progress toward meeting global targets-worldwide. Morb Mortal Wkly Rep. 2018;69(2020):281–5.
Gupta L, Ahmed S, Jain A, Misra R. Emerging role of metabolomics in rheumatology. Int J Rheum Dis. 2018;21:1468–77.
Priori R, Scrivo R, Brandt J, Valerio M, Casadei L, Valesini G, Manetti C. Metabolomics in rheumatic diseases: the potential of an emerging methodology for improved patient diagnosis, prognosis, and treatment efficacy. Autoimmun Rev. 2013;12:1022–30.
The PLOS O.N.E. Correction: Biomarkers of inflammation, immunosuppression and stress are revealed by metabolomic profiling of tuberculosis patients. PLoS One. 2016;11:e0153050.
Zhou A, Ni J, Xu Z, Wang Y, Zhang H, Wu W, Lu S, Karakousis PC, Yao YF. Metabolomics specificity of tuberculosis plasma revealed by 1H NMR spectroscopy. Tuberculosis. 2015;95:294–302.
Zhang A, Sun H, Yan G, Wang P, Wang X. Metabolomics for biomarker discovery: moving to the clinic. BioMed Res Int. 2015;2015:354671.
Serkova NJ, Niemann CU. Pattern recognition and biomarker validation using quantitative 1H-NMR-based metabolomics. Expert Rev Mol Diagn. 2006;6:717–31.
Guleria A, Kumar A, Kumar U, Raj R, Kumar D. NMR based metabolomics: an exquisite and facile method for evaluating therapeutic efficacy and screening drug toxicity. Curr Top Med Chem. 2018;18:1827–49.
Geamanu A, Gupta SV, Bauerfeld C, Samavati L. Metabolomics connects aberrant bioenergetic, transmethylation, and gut microbiota in sarcoidosis. Metabolomics. 2016;12:35.
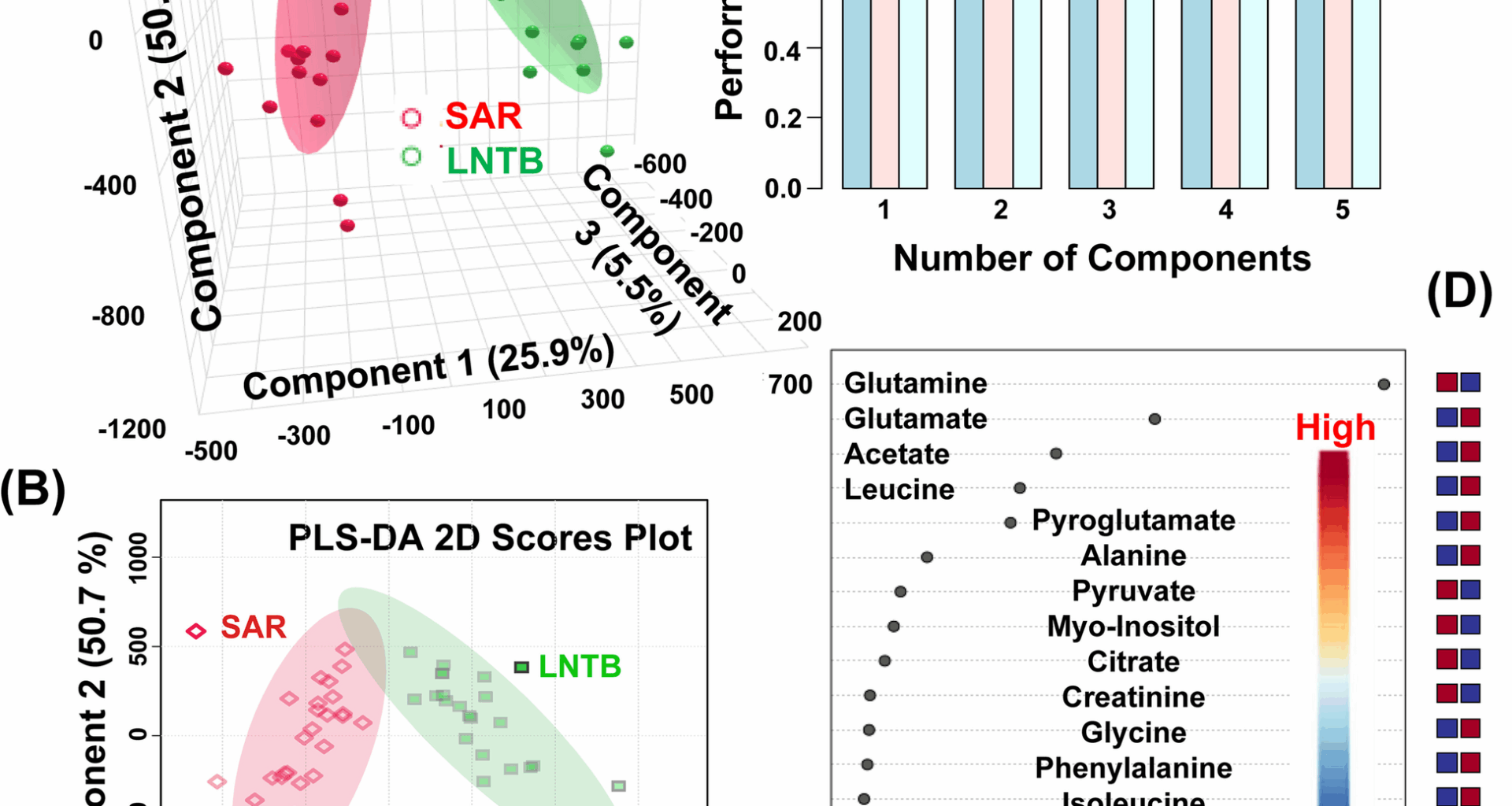
Rai MK, Yadav S, Jain A, Singh K, Kumar A, Raj R, Dubey D, Singh H, Guleria A, Chaturvedi S. Clinical metabolomics by NMR revealed serum metabolic signatures for differentiating sarcoidosis from tuberculosis. Metabolomics. 2023;19:92.
Gupta L, Guleria A, Rawat A, Kumar D, Aggarwal A. NMR-based clinical metabolomics revealed distinctive serum metabolic profiles in patients with spondyloarthritis. Magn Reson Chem. 2021;59:85–98.
Guleria A, Misra DP, Rawat A, Dubey D, Khetrapal CL, Bacon P, Misra R, Kumar D. NMR-based serum metabolomics discriminates Takayasu arteritis from healthy individuals: a proof-of-principle study. J Proteome Res. 2015;14:3372–81.
Guleria A, Pratap A, Dubey D, Rawat A, Chaurasia S, Sukesh E, Phatak S, Ajmani S, Kumar U, Khetrapal CL, Bacon P, Misra R, Kumar D. NMR based serum metabolomics reveals a distinctive signature in patients with Lupus Nephritis. Sci Rep. 2016;6:35309.
Dubey D, Chaurasia S, Guleria A, Kumar S, Modi DR, Misra R, Kumar D. Metabolite assignment of ultrafiltered synovial fluid extracted from knee joints of reactive arthritis patients using high resolution NMR spectroscopy. Magn Reson Chem. 2019;57:30–43.
Kusum K, Raj R, Rai S, Pranjali P, Ashish A, Vicente-Mun¦âoz S, Chaube R, Kumar D. Elevated circulatory proline to glutamine ratio (PQR) in endometriosis and its potential as a diagnostic biomarker. ACS Omega. 2022;7:14856–66.
Yadav S, Kumar A, Singh S, Ahmad S, Singh G, Khan AR, Chaurasia RN, Kumar D. NMR based serum metabolomics revealed metabolic signatures associated with oxidative stress and mitochondrial damage in brain stroke. Metab Brain Dis. 2024;39:283–294.
Singh A, Prakash V, Gupta N, Kumar A, Kant R, Kumar D. Serum metabolic disturbances in lung cancer investigated through an elaborative nmr-based serum metabolomics approach. ACS Omega. 2022;7:5510–20.
Kumar U, Jain A, Guleria A, Misra DP, Goel R, Danda D, Misra R, Kumar D. Circulatory Glutamine/Glucose ratio for evaluating disease activity in Takayasu arteritis: a NMR based serum metabolomics study. J Pharm Biomed Anal. 2020;180:113080.
Muhammed H, Kumar D, Dubey D, Kumar S, Chaurasia S, Guleria A, Majumder S, Singh R, Agarwal V, Misra R. Metabolomics analysis revealed significantly higher synovial Phe/Tyr ratio in reactive arthritis and undifferentiated spondyloarthropathy. Rheumatol. 2020;59(7):1587–1590.
Lee LC, Liong CY, Jemain AA. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: a review of contemporary practice strategies and knowledge gaps. Analyst. 2018;143:3526–39.
Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques P+, Li S, Xia J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–96.
Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–60.
Mahieu B, Qannari EM, Jaillais BT. Extension and significance testing of variable importance in projection (VIP) indices in partial least squares regression and principal components analysis. Chemometr Intell Lab Syst. 2023;242:104986.
Amalia F, Syamsunarno MRA, Triatin RD, Fatimah SN, Chaidir L, Achmad TH. The role of amino acids in tuberculosis infection: a literature review. Metabolites. 2022;12:933.
Cho Y, Park Y, Sim B, Kim J, Lee H, Cho SN, Kang YA, Lee SG. Identification of serum biomarkers for active pulmonary tuberculosis using a targeted metabolomics approach. Sci Rep. 2020;10:3825.
Chen ES, Moller DR. Sarcoidosis-scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–67.
Saboor SA, McFadden JOHN, Johnson NM. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet. 1992;339:1012–5.
Melani AS, Simona A, Armati M, d’Alessandro M, Bargagli E. A Comprehensive review of sarcoidosis diagnosis and monitoring for the pulmonologist. Pulm Ther. 2021;7:309–24.
Park HJ, Im Jung J, Chung MH, Song SW, Kim HL, Baik JH, Han DH, Kim KJ, Lee KY. Typical and atypical manifestations of intrathoracic sarcoidosis. Korean J Radiol. 2009;10:623–31.
Soto-Gomez N, Peters JI, Nambiar AM. Diagnosis and management of sarcoidosis. Am Fam Physician. 2016;93:840–50.
Starshinova A, Zinchenko Y, Filatov M, Denisova N, Istomina E, Landa S, Burdakov V, Churilov L, Sapozhnikova N, Pavlova M. Specific features of immune complexes in patients with sarcoidosis and pulmonary tuberculosis. Immunol Res. 2018;66:737–43.
Agrawal R, Kee AR, Ang L, Hang YT, Gupta V, Kon OM, Mitchell D, Zierhut M, Pavesio C. Tuberculosis or sarcoidosis: opposite ends of the same disease spectrum? Tuberculosis. 2016;98:21–6.
Du Preez I. Can metabolomics improve tuberculosis diagnostics? Metabolomics. 2014;10:0.
Prasse A. The diagnosis, differential diagnosis, and treatment of sarcoidosis. Dtsch Arztebl Int. 2016;113:565.
Bargagli E, Mazzi A, Rottoli P. Markers of inflammation in sarcoidosis: blood, urine, BAL, sputum, and exhaled gas. Clin Chest Med. 2008;29:445–58.
Kruit A, Grutters JC, Gerritsen WB, Kos S, Wodzig WK, van den Bosch JM, Ruven HJ. ACE I/D-corrected Z-scores to identify normal and elevated ACE activity in sarcoidosis. Respir Med. 2007;101:510–5.
Miyoshi S, Hamada H, Kadowaki T, Hamaguchi N, Ito R, Irifune K, Higaki J. Comparative evaluation of serum markers in pulmonary sarcoidosis. Chest. 2010;137:1391–7.
Du SS, Zhao MM, Zhang Y, Zhang P, Hu Y, Wang LS, Zhou Y, Li QH, Li Y, Du YK. Screening for differentially expressed proteins relevant to the differential diagnosis of sarcoidosis and tuberculosis. PLoS ONE. 2015;10:e0132466.
Sodsri T, Baughman RP, Sriprasart T. Diagnosis of pulmonary sarcoidosis in tuberculosis endemic area-a narrative review. J Thorac Dis. 2023;15:5760.
Kim J, Lee S, Moodley Y, Yagnik L, Birnie D, Dwivedi G. The role of the host-microbiome and metabolomics in sarcoidosis. Am J Physiol Cell Physiol. 2023;325:C1336–53.
Mirsaeidi M, Banoei MM, Nienow CK, Abassi T, Hakim A, Schraufnagel D, Winston BW, Sweiss N, Baughman R, Garcia JG. Plasma metabolomic profile in fibrosing pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:29–38.
Banoei MM, Iupe I, Bazaz RD, Campos M, Vogel HJ, Winston BW, Mirsaeidi M. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci Rep. 2019;9:1–12.
Parihar R, Shukla R, Baishya B, Kalita J, Haldar R, Misra UK. NMR based CSF metabolomics in tuberculous meningitis: correlation with clinical and MRI findings. Metabol Brain Dis. 2022;37:773–785.
Kumar D, Raj R, Jain A, Guleria A, Kumar U, Rai MK, Singh H, Chaturvedi S, Nath A, Misra DP. Serum based metabolomics analysis revealed highly sensitive and specific panel of metabolic markers for differential diagnosis of pulmonary sarcoidosis and tuberculosis. F1000Res. 2019;8:304. https://doi.org/10.7490/f1000research.1116481.1.
Magdalena D, Michal S, Marta S, Magdalena KP, Anna P, Magdalena G. Targeted metabolomics analysis of serum and Mycobacterium tuberculosis antigen-stimulated blood cultures of pediatric patients with active and latent tuberculosis. Sci Rep. 2022;12:4131.
Fujimoto M, Matsumoto T, Serada S, Tsujimura Y, Hashimoto S, Yasutomi Y, Naka T. Leucine-rich alpha 2 glycoprotein is a new marker for active disease of tuberculosis. Sci Rep. 2020;10:3384.