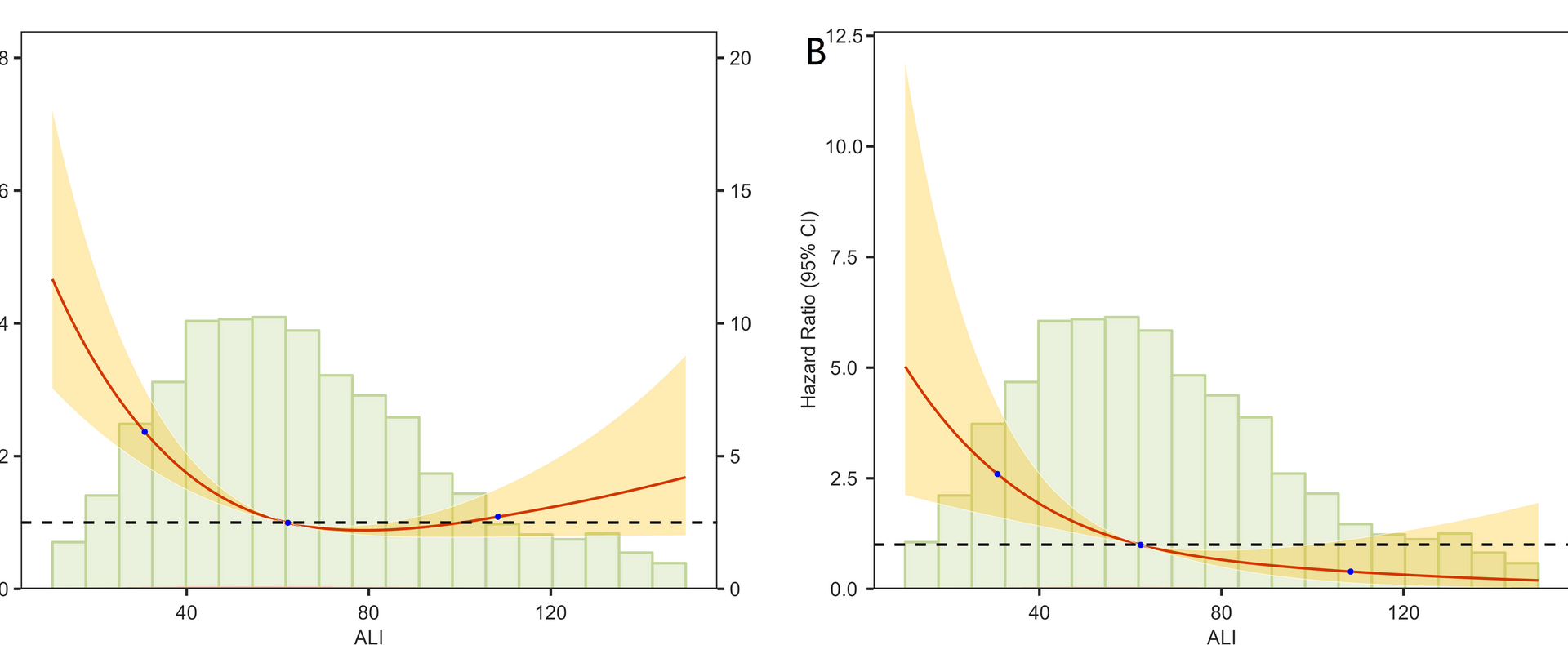
This study establishes the Advanced Lung Cancer Inflammation Index (ALI) as a robust predictor of all-cause and cardiovascular mortality in hypertensive patients, revealing a significant inverse association wherein the highest ALI quartile (Q4) demonstrated 53.8% lower all-cause mortality and 83.5% lower cardiovascular mortality versus the lowest quartile (Q1). These dose-dependent relationships persisted across progressively adjusted models, with strong predictive accuracy (AUC: 0.735–0.772) evidenced by time-dependent ROC analysis over 1–5 years. Decision Curve Analysis further confirmed ALI’s superior clinical utility across threshold probabilities.
Our findings align with prior prognostic research: Chen et al. (2023) reported ALI-associated mortality reduction in type 2 diabetes through inflammation-nutrition pathways, while Zhang et al. (2022) and Tu et al. (2023) established ALI’s cardiovascular predictive value in hypertension. This study extends such evidence through methodological advancements—including nationally representative sampling (NHANES, N = 2,805), extended median follow-up (57.6 months), and competing risk models with DCA—strengthening ALI’s clinical validity for addressing competing mortality.
When exploring the potential mechanisms linking the ALI to mortality in hypertensive patients, it is critical to integrate the biological properties of its components—BMI, Alb, and the NLR—with the pathophysiological processes of hypertension. ALI inherently reflects systemic inflammation and nutritional-metabolic balance, both of which are pivotal drivers of hypertension progression and its complications [28, 29]. Elevated NLR indicates systemic inflammatory activation, where neutrophils release reactive oxygen species (ROS) and pro-inflammatory cytokines (e.g., IL-6, TNF-α), exacerbating endothelial dysfunction, oxidative stress, and atherosclerotic plaque formation [30]. Concurrently, reduced lymphocyte counts may reflect immune dysregulation, impairing anti-inflammatory and reparative capacities, thereby accelerating vascular remodeling and myocardial fibrosis [31].
Alb, a dual biomarker of nutrition and inflammation, influences prognosis through multiple pathways. Albumin exhibits antioxidant and anti-inflammatory properties by binding pro-inflammatory molecules (e.g., free fatty acids, lipopolysaccharides), thereby mitigating endothelial injury [32]. Additionally, low Alb levels correlate with increased vascular permeability and microcirculatory impairment, potentially hastening ischemic damage to target organs (e.g., heart, kidneys) [33]. In hypertensive patients, chronic pressure overload and renin-angiotensin system activation may induce proteinuria and Alb depletion, perpetuating a vicious cycle [34,35,36].
BMI, as a component of ALI, exhibits a nonlinear relationship with outcomes. While obesity (high BMI) is linked to insulin resistance and metabolic syndrome, the improved prognosis observed with higher ALI values (which include BMI) in this study may align with the “obesity paradox”—moderate adiposity might confer protective effects in chronic diseases by providing energy reserves and buffering inflammatory responses [37]. However, this paradox underscores that ALI does not merely reflect adiposity but integrates nutritional status (Alb) and inflammation (NLR) into a comprehensive risk assessment. For instance, high BMI combined with low Alb or elevated NLR may indicate sarcopenic obesity, where metabolic disturbances and inflammatory synergism markedly elevate mortality risk [38].
The multidimensional integration inherent to ALI enhances its representation of hypertension’s pathophysiological networks, where the interplay between inflammation and malnutrition accelerates multi-organ dysfunction. Specifically, inflammatory mediators exacerbate blood pressure variability and cardiac remodeling through renin-angiotensin-aldosterone system (RAAS) and sympathetic tone activation, while concurrent malnutrition compromises myocardial energy metabolism and tissue repair—collectively hastening heart failure progression. By quantifying this synergistic interaction, ALI provides a distinctive analytical framework for identifying high-risk subpopulations.
The dynamic and modifiable nature of the components of ALI further enhances its clinical appeal. Our findings suggest that ALI may not only be a prognostic marker but also a potential surrogate for monitoring the efficacy of interventions. For instance, pharmacological interventions commonly used in hypertensive patients, such as ACE inhibitors/ARBs or SGLT2 inhibitors, often possess pleiotropic anti-inflammatory effects beyond blood pressure control. These drugs can reduce systemic inflammation, lower neutrophil count, and modulate immune function, which directly decreases the Neutrophil-to-Lymphocyte Ratio (NLR)—the most significant mechanism through which such interventions could potentially increase ALI. Similarly, exercise and lifestyle modifications can improve ALI through multiple pathways: exercise exerts potent anti-inflammatory effects, reducing chronic low-grade inflammation and improving immune regulation to lower NLR; it also improves body composition by reducing adiposity and increasing lean mass, favorably affecting BMI; and concomitant dietary improvements can enhance nutritional status and serum albumin levels.
Future investigations utilizing animal models should aim to elucidate ALI’s associations with specific molecular pathways—such as NLRP3 inflammasome activation and lipid peroxidation products—and employ multi-omics technologies to delineate ALI-related metabolic-inflammatory networks [39]. This mechanistic exploration could inform targeted interventions; for instance, combined anti-inflammatory therapies (e.g., IL-1β inhibitors) with nutritional support in low-ALI patients might disrupt pathological cascades to improve outcomes. Such insights would substantiate ALI’s translational value, catalyzing a paradigm shift in hypertension management from isolated blood pressure control toward multidimensional risk modulation.
The predictive power of ALI stems from its integration of inflammatory and metabolic pathways, positioning it as a holistic stratification tool. Clinically, ALI’s simplicity supports routine implementation; patients with ALI < 44.02 (Q1) represent candidates for intensified anti-inflammatory or nutritional interventions, though prospective validation is warranted. Future research should explore molecular mechanisms (e.g., NLRP3 inflammasome) via omics technologies, validate findings ethnically, and test targeted therapies (e.g., IL-1β inhibitors with nutritional support) to advance hypertension management toward multidimensional risk modulation.
Hypertension is a multifactorial disorder with diverse etiologies, clinical presentations, and comorbid backgrounds, which may influence the prognostic utility of inflammatory-nutritional biomarkers such as ALI. For instance, certain hypertension subtypes—such as resistant hypertension, hypertension with concomitant metabolic syndrome, or those with overt inflammatory comorbidities—may exhibit heightened sensitivity to ALI alterations due to more pronounced interplay between inflammation, malnutrition, and cardiovascular risk. It is important to note that while our study establishes ALI as a strong predictor of mortality, further research is needed to explore whether a causal relationship exists between ALI and improved survival outcomes. The integration of nutritional and inflammatory pathways captured by ALI suggests it may reflect a modifiable physiological state. To rigorously evaluate whether improving ALI directly reduces mortality risk, future studies should employ causal inference frameworks such as target trial emulation. This methodology applies the principled design of randomized controlled trials—including explicit eligibility criteria, treatment strategies, and outcome definitions—to analyze observational data, thereby strengthening causal interpretations. Applying such approaches would help clarify whether ALI represents a promising target for therapeutic interventions aimed at enhancing survival in hypertensive patients [40].
This study possesses notable strengths including a substantial sample size, rigorous adjustment for confounders (e.g., diabetes, smoking status), and application of survey-weighted analyses to ensure national representativeness. However, several limitations warrant careful consideration. First, residual confounding from unmeasured factors such as medication adherence may persist despite comprehensive multivariable adjustments. Second, the absence of detailed hypertension severity data (e.g., staging or treatment compliance) in NHANES restricts our ability to examine ALI’s prognostic role across disease progression spectra. Third, generalizability is constrained by the exclusively U.S.-based cohort, necessitating external validation in diverse ethnic populations. Fourth, methodological constraints emerge from the limited cardiovascular mortality events (n = 89) relative to covariates in fully adjusted models, introducing potential overfitting and sparse-data bias that may compromise precision in extreme hazard ratio estimates (e.g., the 83.5% CVD mortality reduction in Q4). Although survey-weighted Cox regression provides population-representative estimates, the complex interaction between limited events and multiple confounders demands cautious interpretation of point estimates; future validation should employ penalized regression techniques (e.g., Firth’s correction) while maintaining the 10–15 events-per-variable threshold. Fifth, while ALI demonstrates promising predictive capability, reported discrimination metrics (e.g., 1-year all-cause mortality AUC = 0.772) require cautious interpretation due to the absence of internal validation (bootstrapping/split-sample methods) and AUC confidence intervals – particularly given potential survey weighting effects that might inflate performance estimates. Future studies should implement robust validation frameworks with appropriate weighting adjustments. Additionally, our complete-case analytical approach excluding records with missing covariates (n = 9,620) and ALI outliers (n = 123) may introduce selection bias, potentially amplifying observed dose-response relationships and predictive performance; more sophisticated missing data handling (e.g., multiple imputation) should be prioritized in subsequent research. Finally, we acknowledge that important clinical covariates such as the duration of hypertension, chronic obstructive pulmonary disease (COPD), chronic kidney disease, and other cardiovascular conditions (e.g., myocardial infarction, stroke) were not included in our multivariate models or subgroup analyses. These factors are strongly associated with hypertensive outcomes and could potentially confound the observed associations. Although such data were not consistently available across NHANES cycles, their omission may limit the comprehensiveness of risk adjustment. Future studies should prioritize the inclusion of these clinically relevant variables to enhance the robustness and clinical applicability of prognostic models involving ALI.