Reposted from U of U Health.
 Paul Sigala. Photo credit: Charlie Ehlert / University of Utah Health
Paul Sigala. Photo credit: Charlie Ehlert / University of Utah Health
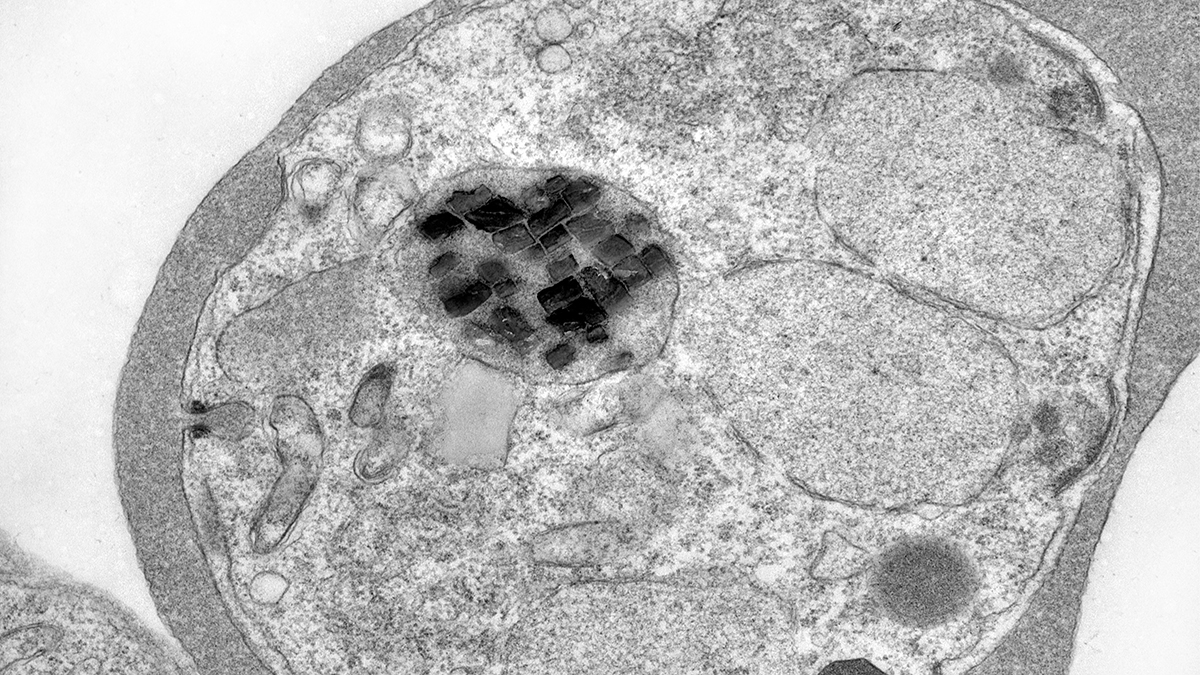
Every cell of the deadly Plasmodium falciparum parasite, the organism that causes malaria, contains a tiny compartment full of microscopic iron crystals. As long as the parasite is alive, the crystals dance. They spin, jolt and ricochet in their little bubble like change in an overclocked washing machine, too fast and chaotic to even be tracked by traditional scientific techniques. And when the parasite dies, they stop.
The iron crystals have long been an important target for antimalarial drugs, but their motion has mystified scientists since it was first detected.
“People don’t talk about what they don’t understand, and because the motion of these crystals is so mysterious and bizarre, it’s been a blind spot for parasitology for decades,” said Paul Sigala, associate professor of biochemistry in the Spencer Fox Eccles School of Medicine at the University of Utah.
Now, Sigala’s research team, in collaboration with Utah’s Price College of Engineering, has finally found what makes the crystals dance: the same chemical reaction that powers spacefaring rockets. The findings, published Oct. 28, 2025, in PNAS, could reveal new targets for malaria treatments and provide new insights for creating nanoscale robots.
Biological rocket fuel
 Erica Hastings working with malaria parasites in lab. Photo credit: Aldo Garcia Guerrero.
Erica Hastings working with malaria parasites in lab. Photo credit: Aldo Garcia Guerrero.
The crystals, which are made of an iron-based compound called heme, move by triggering the breakdown of hydrogen peroxide into water and oxygen, the researchers discovered. The reaction releases energy, giving the crystals the “kick” they need to spin into motion.
It’s a form of propulsion common in aerospace engineering, where peroxide fuel launches satellites into orbit, but previously unknown in biology. “This hydrogen peroxide decomposition has been used to power large-scale rockets,” said Erica Hastings, postdoctoral researcher in biochemistry in the School of Medicine. “But I don’t think it has ever been observed in biological systems.”
Hydrogen peroxide is found at high levels inside the microscopic compartment that contains iron crystals, and parasites produce the compound as a waste product, so it had stood out to the researchers as a potential chemical fuel that might power the crystals’ motion. Indeed, the scientists found that hydrogen peroxide on its own was enough to set purified crystals spinning—no parasite required.
Conversely, when the researchers raised malaria parasites at unusually low levels of oxygen, which lowers the amount of peroxide the parasites produce, the crystals decelerated to about half their normal speed, even though the parasites were otherwise healthy.
Crystal motion may aid parasite survival
The researchers suspect that the frenetic motion of the crystals may be important for malaria parasites to stay alive, and they have a few ideas why. Peroxide itself is extremely toxic to cells. The spinning crystals might be a way for the malaria organism to “burn off” excess toxic peroxide before it can cause harmful chemical reactions and damage the parasite.
Sigala adds that the spinning motion might also help the parasite quickly deal with excess heme by keeping crystals from clumping together. Clumped-up crystals would prevent the parasite from storing additional heme as quickly, because they’d have less available surface to add new heme to. By keeping the crystals in constant motion, the malaria parasite may ensure that it’s able to sequester additional heme efficiently.
Powering new robots and new drugs
The spinning crystals are the first known example in biology of a self-propelled metallic nanoparticle, the researchers said. But they suspect that this phenomenon is much more widespread. The new findings could inspire improved designs for microscopic robots, the researchers added.
“Nano-engineered self-propelling particles can be used for a variety of industrial and drug-delivery applications, and we think there are potential insights that will come from these results,” Sigala said.
The results could also eventually lead to better antimalarial drugs.
“We think that the breakdown of hydrogen peroxide likely makes an important contribution to reducing cellular stress,” Sigala said. “If there are ways to block the chemistry at the crystal surface, that alone might be sufficient to kill parasites.”
Their tiny chemical rockets are wildly different from any known aspect of human biology—and that means that they’re a powerful potential drug target. Drugs that target such a parasite-unique mechanism are much less likely to have dangerous side effects.
“If we target a drug to an area that’s very different from human cells, then it’s probably not going to have extreme side effects,” Hastings explained. “If we can define how this parasite is different from our bodies, it gives us access to new directions for medications.”
The results were published Oct. 28 in PNAS as “Chemical propulsion of hemozoin crystal motion in malaria parasites.” Co-authors include members of the Huntsman Cancer Institute and the Price College of Engineering’s departments of Biomedical Engineering and Mechanical Engineering and Scientific Computing and Imaging Institute. The work was supported by the National Institutes of Health, the Utah Center for Iron & Heme Disorders, the Price College of Engineering and 3i Initiative at University of Utah Health. Content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Banner depicts an electron microscope image of a red blood cell (largest blob) infected with a malaria parasite (slightly smaller circle). Dark, brick-like shapes are iron crystals. Microscope video shows a live malaria parasite full of jittering iron crystals. Video and image credit: Erica Hastings.
MEDIA & PR CONTACTS