FAO. The State of Food Security and Nutrition in the World 2022. FAO; IFAD; UNICEF ; WFP; WHO, editor. The State of Food Security and Nutrition in the World 2022. Rome, Italy: FAO; 2022.
Kumar M, Ahmad S, Singh RP. Plant growth promoting microbes: Diverse roles for sustainable and ecofriendly agriculture. Energy Nexus. 2022;7:100133.
Matías J, Rodríguez MJ, Carrillo-Vico A, Casals J, Fondevilla S, Haros CM, et al. From ‘Farm to Fork’: exploring the potential of nutrient-rich and stress-resilient emergent crops for sustainable and healthy food in the Mediterranean region in the face of climate change challenges. Plants. 2024.
Vernooy R. Does crop diversification lead to climate-related resilience? Improving the theory through insights on practice. Agroecol Sustain Food Syst. 2022;46:877–901.
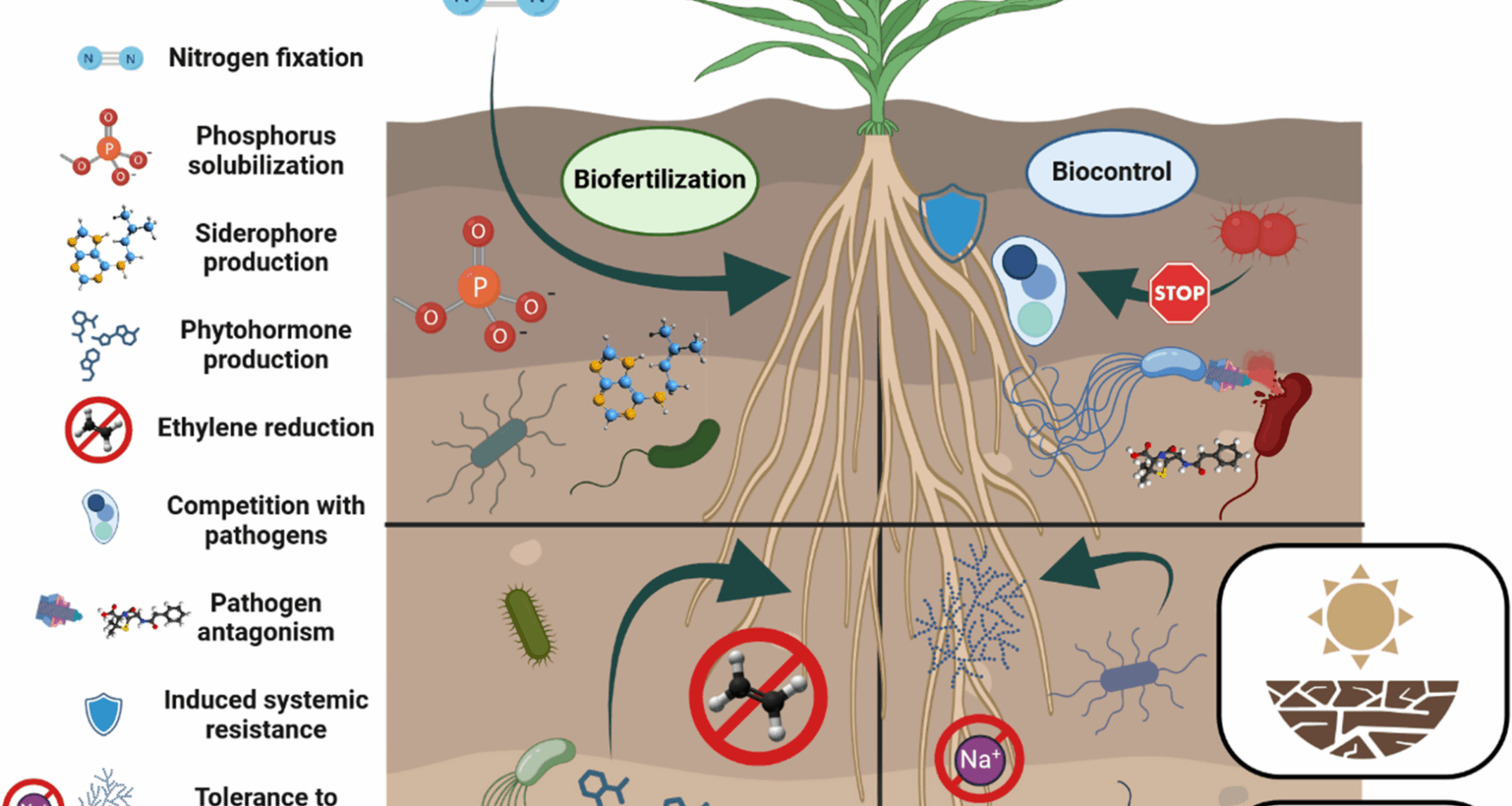
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;871:402666.
García-Fraile P, Menéndez E, Rivas R. Role of bacterial biofertilizers in agriculture and forestry. Aims Bioeng. 2015;2(3):183–205.
Dastogeer KMG, Tumpa FH, Sultana A, Akter MA, Chakraborty A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr Plant Biol. 2020;23:100161.
Bandopadhyay S, Li X, Bowsher AW, Last RL, Shade A. Disentangling plant- and environment-mediated drivers of active rhizosphere bacterial community dynamics during short-term drought. Nat Commun. 2024;15:1–16.
Di Benedetto NA, Corbo MR, Campaniello D, Cataldi MP, Bevilacqua A, Sinigaglia M, et al. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: a focus on wheat. AIMS Microbiol. 2017;3:413.
Khan N, Mehmood A. Revisiting climate change impacts on plant growth and its mitigation with plant growth promoting rhizobacteria. South Afr J Bot. 2023;160:586–601.
Shah A, Nazari M, Antar M, Msimbira LA, Naamala J, Lyu D, et al. PGPR in agriculture: a sustainable approach to increasing climate change resilience. Front Sustain Food Syst. 2021;5:667546.
Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci Total Environ. 2020;743:140682.
Pérez-Montaño F, Alías-Villegas C, Bellogín RA, Del Cerro P, Espuny MR, Jiménez-Guerrero I, et al. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res. 2014;169(5–6):325–36.
Benitez-Alfonso Y, Soanes BK, Zimba S, Sinanaj B, German L, Sharma V, et al. Enhancing climate change resilience in agricultural crops. Curr Biol. 2023;33:R1246–61.
de Oliveira Lopes ÁL, Setubal IS, da Costa Neto VP, Zilli JE, Rodrigues AC, Bonifacio A. Synergism of Bradyrhizobium and Azospirillum baldaniorum improves growth and symbiotic performance in lima bean under salinity by positive modulations in leaf nitrogen compounds. Appl Soil Ecol. 2022;180:104603.
Zimmermann SD, Roussillon L, Mandon K, Oresnik IJ, Hawkins JP. The rhizobium-legume symbiosis: co-opting successful stress management. Front Plant Sci. 2022;12:796045.
Latt ZK, Thant S, Aung NN, Aye OM, Oo NN, Htun TMM, et al. Phosphate solubilization of Bacillus megaterium isolated from non-saline soils under salt stressed conditions. Journal of Bacteriology & Mycology: Open Access. 2018;6:335–41.
Chen W, Yang F, Zhang L, Wang J. Organic acid secretion and phosphate solubilizing efficiency of Pseudomonas sp. PSB12: effects of phosphorus forms and carbon sources. Geomicrobiol J. 2016;33:870–7.
Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27(5):637–57.
Trapet P, Avoscan L, Klinguer A, Pateyron S, Citerne S, Chervin C, et al. The Pseudomonas fluorescens siderophore pyoverdine weakens Arabidopsis thaliana defense in favor of growth in iron-deficient conditions. Plant Physiol. 2016;171:675–93.
Chandra D, Srivastava R, Sharma AK. Influence of IAA and ACC deaminase producing fluorescent pseudomonads in alleviating drought stress in wheat (Triticum aestivum). Agric Res. 2018;7:290–9.
Kang SM, Shahzad R, Bilal S, Khan AL, Park YG, Lee KE, et al. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019.
Egamberdieva D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol Plant. 2009;31:861–4.
Matsuda R, Handayani ML, Sasaki H, Takechi K, Takano H, Takio S. Production of indoleacetic acid by strains of the epiphytic bacteria Neptunomonas spp. isolated from the red alga Pyropia yezoensis and the seagrass Zostera marina. Arch Microbiol. 2018;200:255–65.
Kang S-M, Latif Khan A, Waqas M, Asaf S, Lee K-E, Park Y-G, et al. Bacillus tequilensis SSB07 induced thermotolerance in soybean. J Plant Interact. 2019;14:416–23.
Kang S-M, Latif Khan A, Hamayun M, Hussain J, Joo G-J, You Y-H, et al. Gibberellin-Producing Promicromonospora sp. SE188 Improves Solanum lycopersicum Plant Growth and Influences Endogenous Plant Hormones. J Microbiology. 2012;50:902–9.
Patel T, Saraf M. Biosynthesis of phytohormones from novel rhizobacterial isolates and their in vitro plant growth-promoting efficacy. J Plant Interact. 2017;12:480–7.
Park Y-G, Mun B-G, Kang S-M, Hussain A, Shahzad R, Seo C-W, et al. Bacillusaryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS One. 2017;12(3):e0173203.
Kavi Kishor PB, Tiozon RN, Fernie AR, Sreenivasulu N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022;27:1283–95.
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep. 2016;6:34768.
Tiwari S, Lata C, Chauhan PS, Nautiyal CS. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem. 2016;99:108–17.
Arkhipova T, Martynenko E, Sharipova G, Kuzmina L, Ivanov I, Garipova M, et al. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants. 2020;9:1429.
Han Y, Wang R, Yang Z, Zhan Y, Ma Y, Ping S, et al. 1-aminocyclopropane-1-carboxylate deaminase from Pseudomonas stutzeri A1501 facilitates the growth of rice in the presence of salt or heavy metals. J Microbiol Biotechnol. 2015;25:1119–28.
Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105 OPEN. Sci Rep. 2018;8:1950
Bal HB, Adhya TK. Alleviation of submergence stress in rice seedlings by plant growth-promoting rhizobacteria with ACC deaminase activity. Front Sustain Food Syst. 2021;5:606158.
Gu S, Wei Z, Shao Z, Friman VP, Cao K, Yang T, et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat Microbiol. 2020;5:1002–10.
Goswami D, Thakker JN, Dhandhukia PC. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric Informa Healthcare. 2016;2:1127500.
Kavino M, Harish S, Kumar N, Saravanakumar D, Samiyappan R. Effect of chitinolytic PGPR on growth, yield and physiological attributes of banana (Musa spp.) under field conditions. Appl Soil Ecol. 2010;45:71–7.
Sharifi R, Ryu CM. Sniffing bacterial volatile compounds for healthier plants. Curr Opin Plant Biol. 2018;44:88–97.
Kwak YS, Bonsall RF, Okubara PA, Paulitz TC, Thomashow LS, Weller DM. Factors impacting the activity of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens against take-all of wheat. Soil Biol Biochem. 2012;54:48–56.
Harmsen N, Vesga P, Glauser G, Klötzli F, Heiman CM, Altenried A, et al. Natural plant disease suppressiveness in soils extends to insect pest control. Microbiome. 2024;12:1–16.
Maurhofer M, Reimmann C, Schmidli-Sacherer P, Heeb S, Haas D, Défago G. Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco against tobacco necrosis virus. Phytopathology. 1998;88:678–84.
De Vleesschauwer D, Cornelis P, Höfte M. Redox-active pyocyanin secreted by Pseudomonas aeruginosa 7NSK2 triggers systemic resistance to Magnaporthe grisea but enhances Rhizoctonia solani susceptibility in rice. Mol Plant-Microbe Interact. 2006;19:1406–19.
Naseem H, Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact. 2014;9:689–701.
Batool T, Ali S, Seleiman MF, Naveed NH, Ali A, Ahmed K, et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci Rep. 2020.
Zhang H, Kim M-S, Sun Y, Dowd SE, Shi H, Paré PW. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant Microbe Interact. 2008;21:737–44.
Jiménez-Gómez A, García-Estévez I, García-Fraile P, Escribano-Bailón MT, Rivas R. Increase in phenolic compounds of Coriandrum sativum L. after the application of a Bacillus halotolerans biofertilizer. J Sci Food Agric. 2020;100:2742–9.
Raklami A, Oufdou K, Tahiri AI, Mateos-Naranjo E, Navarro-Torre S, Rodríguez-Llorente ID, et al. Safe cultivation of Medicago sativa in metal-polluted soils from semi-arid regions assisted by heat-and metallo-resistant PGPR. Microorganisms. 2019.
Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo). 2012;2012:1–15.
Cosentino SL, Testa G, Scordia D, Copani V. Sowing time and prediction of flowering of different hemp (Cannabis sativa L.) genotypes in southern Europe. Ind Crops Prod. 2012;37:20–33.
Morte A, Kagan-Zur V, Navarro-Ródenas A, Sitrit Y. Cultivation of desert truffles—A crop suitable for arid and semi-arid zones. Agronomy. 2021;11(8):1462.
Lucas MM, Stoddard FL, Annicchiarico P, Frías J, Martínez-Villaluenga C, Sussmann D, et al. The future of lupin as a protein crop in Europe. Front Plant Sci. 2015;6:160197.
Quiñones MA, Lucas MM, Pueyo JJ. Adaptive mechanisms make lupin a choice crop for acidic soils affected by aluminum toxicity. Front Plant Sci. 2022.
Quiñones MA, Fajardo S, Fernández-Pascual M, Lucas MM, Pueyo JJ. Nodulated white lupin plants growing in contaminated soils accumulate unusually high mercury concentrations in their nodules, roots and especially cluster roots. Horticulturae. 2021;7:302.
Msaddak A, Mars M, Quiñones MA, Lucas MM, Pueyo JJ. Lupin, a unique legume that is nodulated by multiple microsymbionts: the role of horizontal gene transfer. Int J Mol Sci. 2023;24:6496.
Aslam MM, Pueyo JJ, Pang J, Yang J, Chen W, Chen H, et al. Root acid phosphatases and rhizobacteria synergistically enhance white lupin and rice phosphorus acquisition. Plant Physiol. 2022;190:2449–65.
Ferchichi N, Toukabri W, Vrhovsek U, Angeli A, Masuero D, Mhamdi R, et al. Inoculation of Lupinus albus with the nodule-endophyte Paenibacillus glycanilyticus LJ121 improves grain nutritional quality. Arch Microbiol. 2020;202:283–91.
Ferchichi N, Toukabri W, Boularess M, Smaoui A, Mhamdi R, Trabelsi D. Isolation, identification and plant growth promotion ability of endophytic bacteria associated with lupine root nodule grown in Tunisian soil. Arch Microbiol. 2019;201:1333–49.
Sulewska H, Ratajczak K, Niewiadomska A, Panasiewicz K. The use of microorganisms as bio-fertilizers in the cultivation of white lupine. Open Chem. 2019;17:813–22.
Waraczewska Z, Niewiadomska A, Wolna-Maruwka A, Sulewska H, Budka A, Pilarska AA. The effect of in vitro coinoculation on the physiological parameters of white lupine plants (Lupinus albus L.). Appl Sci. 2022.
González D, Blanco C, Probanza A, Jiménez PA, Robas M. Evaluation of the pgpr capacity of four bacterial strains and their mixtures, tested on Lupinus albus var. Dorado seedlings, for the bioremediation of mercury-polluted soils. Processes. 2021;9:1293.
González-Reguero D, Robas-Mora M, Probanza A, Jiménez PA. Evaluation of the oxidative stress alleviation in Lupinus albus var. Orden Dorado by the inoculation of four plant growth-promoting bacteria and their mixtures in mercury-polluted soils. Front Microbiol. 2022;13:907557.
Gutiérrez Mañero FJ, Probanza A, Ramos B, Colón Flores JJ, Lucas García JA. Effects of culture filtrates of rhizobacteria isolated from wild lupine on germination, growth, and biological nitrogen fixation of lupine seedlings. J Plant Nutr. 2003;26:1101–15.
Hamada MA, Soliman ERS. Characterization and genomics identification of key genes involved in denitrification-DNRA-nitrification pathway of plant growth-promoting rhizobacteria (Serratia marcescens OK482790). BMC Microbiol. 2023;23:210.
Hewedy M, Abdel-Wahab AF, El Mokadem MT, El-Sayed SY. Evaluation of some plant growth promoting rhizobacteria in bioprotecting lupine from infection by Fusarium solani. Egypt J Pest Control. 2011;21:227–32.
Lucas García JA, Probanza A, Ramos B, Colón Flores JJ, Gutiérrez Mañero FJ. Effects of plant growth promoting rhizobacteria (PGPRs) on the biological nitrogen fixation, nodulation, and growth of Lupinus albus 1. cv. Multolupa. Eng Life Sci. 2004;4:71–7.
Mghazli N, Bruneel O, Zouagui R, Hakkou R, Sbabou L. Characterization of plant growth promoting activities of indigenous bacteria of phosphate mine wastes, a first step toward revegetation. Front Microbiol. 2022;13:1026991.
Robas Mora M, Fernández Pastrana VM, Oliva LLG, Lobo AP, Jiménez Gómez PA. Plant growth promotion of the forage plant Lupinus albus Var. Orden Dorado using Pseudomonas agronomica sp. nov. and Bacillus pretiosus sp. nov. added over a valorized agricultural biowaste. Front Microbiol. 2023;13:65–76.
Sarmiento LH, Díaz PM, Dávalos JJ. Caracterización y evaluación del potencial PGPR de la microflora asociada al cultivo de tarwi (Lupinus mutabilis Sweet). Ecol Apl. 2020;19:65–76.
Unno Y, Okubo K, Wasaki J, Shinano T, Osaki M. Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of lupin analysed by phytate utilization ability. Environ Microbiol. 2005;7:396–404.
Weisskopf L, Heller S, Eberl L. Burkholderia species are major inhabitants of white lupin cluster roots. Appl Environ Microbiol. 2011;77:7715–20.
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot. 2006;98:693–713.
Pueyo JJ, Quiñones MA, de la Coba Peña T, Fedorova EE, Lucas MM. Nitrogen and phosphorus interplay in lupin root nodules and cluster roots. Front Plant Sci. 2021;12:644218.
Richardson AE. Regulating the phosphorus nutrition of plants: molecular biology meeting agronomic needs. Plant Soil. 2009;322:17–24.
Lamont BB, Pérez-Fernández M, Rodríguez-Sánchez J. Soil bacteria hold the key to root cluster formation. New Phytol. 2014;206:1156–62.
Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4.
Vocciante M, Franchi E, Fusini D, Pedron F, Barbafieri M, Petruzzelli G, et al. Sustainable recovery of an agricultural area impacted by an oil spill using enhanced phytoremediation. Appl Sci. 2024;14:582.
Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013;37:634–63.
Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–50.
Msaddak A, Quiñones MA, Mars M, Pueyo JJ. The beneficial effects of inoculation with selected nodule-associated PGPR on white lupin are comparable to those of inoculation with symbiotic rhizobia. Plants. 2023.
Kasule F, Diack O, Mbaye M, Kakeeto R, Econopouly BF. Genomic resources, opportunities, and prospects for accelerated improvement of millets. Theoretical and Applied Genetics. 2024;137(12):273.
Kumari D, Thakur N, Upmanyu S, Thakur N, Upmanyu S. The world of millets: a comprehensive overview of millets and their significance citation: the world of millets: a comprehensive overview of Millets and their Significance. J Cereal Res. 2024;16:239–45.
Kheya SA, Talukder SK, Datta P, Yeasmin S, Rashid MH, Hasan AK, et al. Millets: the future crops for the tropics—status, challenges and future prospects. Heliyon. 2023;9(11):e22123.
Yadav OP, Singh DV, Kumari V, Prasad M, Seni S, Singh RK, et al. Production and cultivation dynamics of millets in India. Crop Sci. 2024;64(5):2459–84.
Shivashakarappa K, Gunnaiah R, Ajjappala BS, Kadi A, Vuppula A. Effect of plant growth promoting rhizobacteria on the growth and yield of foxtail millet (Setaria italica L. Beauv). Int J Plant Soil Sci. 2022;34(22):1737–44.
Niu X, Song L, Xiao Y, Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid and their potential in alleviating drought stress. Front Microbiol. 2018;8: 2580.
Swamy CT. Plant growth-promoting rhizobacteria and millets: a sustainable solution for food security. J Drug Res Ayurvedic Sci. 2023;8(Suppl 1):S115–20.
Murali M, Singh SB, Gowtham HG, Shilpa N, Prasad M, Aiyaz M, et al. Induction of drought tolerance in Pennisetum glaucum by ACC deaminase producing PGPR- Bacillus amyloliquefaciens through antioxidant defense system. Microbiol Res. 2021;253:126891.
Tian L, Wang Y, Yang J, Zhang L, Feng B. Rhizosphere bacterial community structure of three minor grain crops: a case-study from paired field sites in northern China. Land Degrad Dev. 2022;33:104–16.
Saritha M, Naorem A, Kumar S, Meena KK, Panwar NR. Exploring the role of microorganisms in enhancing pearl millet growth and productivity. Ann Arid Zone. 2023;62(1):19–27.
Suharno AQ, Sancayaningsih RP, Kasiamdari RS, Soetarto ES. The growth response of pokem (Setaria italica L.) inoculated with arbuscular mycorrhizal fungi (AMF) from tailings area. J Degraded Min Lands Manage. 2021;8(4):2873.
Muthukumar T, Koshila Ravi R. Biodiversity of arbuscular mycorrhizal fungi and its impact on millets growth. Singapore: Springer Nature Singapore; 2023. p. 35–82.
Chang OC, Lin WY. Variation of growth and transcriptome responses to arbuscular mycorrhizal symbiosis in different foxtail millet lines. Bot Stud. 2023;64(1):16.
Fabbrin EG, Gogorcena Y, Mogor AF, Garmendia I, Goicoechea N. Pearl millet growth and biochemical alterations determined by mycorrhizal inoculation, water availability and atmospheric CO2 concentration. Crop Pasture Sci. 2015.
Kamali S, Mehraban A. Effects of nitroxin and arbuscular mycorrhizal fungi on the agro-physiological traits and grain yield of sorghum (Sorghum bicolor L.) under drought stress conditions. PLoS One. 2021;15(12):e0243824.
Ndeko AB, Founoune-Mboup H, Kane A, Cournac L. Arbuscular mycorrhizal fungi alleviate the negative effect of temperature stress in millet lines with contrasting soil aggregation potential. Gesunde Pflanz. 2022;74(1):53–67.
McPartland JM, Guy GW, Hegman W. Cannabis is indigenous to Europe and cultivation began during the Copper or Bronze age: a probabilistic synthesis of fossil pollen studies. Veg Hist Archaeobot. Springer: New York LLC; 2018. p. 635–48.
Small E. Cannabis: a complete guide. 1st ed. Cannabis: A Complete Guide. CRC Press; 2017.
Fike J. Industrial hemp: renewed opportunities for an ancient crop. Crit Rev Plant Sci. 2016.
Winston ME, Hampton-Marcell J, Zarraonaindia I, Owens SM, Moreau CS, Gilbert JA, et al. Understanding cultivar-specificity and soil determinants of the Cannabis microbiome. PLoS One. 2014;9(6): e99641.
Comeau D, Balthazar C, Novinscak A, Bouhamdani N, Joly DL, Filion M. Interactions between Bacillus Spp., Pseudomonas Spp. and Cannabis sativa promote plant growth. Front Microbiol. 2021;12:715758.
Comeau D, Novinscak A, Joly DL, Filion M. Spatio-temporal and cultivar-dependent variations in the cannabis microbiome. Front Microbiol. 2020;11: 491.
Conant RT, Walsh RP, Walsh M, Bell CW, Wallenstein MD. Effects of a microbial biostimulant, mammoth PTM, on Cannabis sativa Bud Yield. J Hortic. 2017;4(191):2376–354.
Balthazar C, Novinscak A, Cantin G, Joly DL, Filion M. Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis cinerea and other Cannabis fungal pathogens. Phytopathology. 2022;112:549–60.
Pagnani G, Pellegrini M, Galieni A, D’Egidio S, Matteucci F, Ricci A, et al. Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: An alternative fertilization strategy to improve plant growth and quality characteristics. Ind Crops Prod. 2018;123:75–83.
Afzal I, Shinwari ZK, Iqrar I. Selective isolation and characterization of agriculturally beneficial endophytic bacteria from wild hemp using canola. Pak J Bot. 2015;47(5):1999–2008.
Scott C, Punja ZK. Biological control of Fusarium oxysporum causing damping-off and Pythium myriotylum causing root and crown rot on cannabis (Cannabis sativa L.) plants. Can J Plant Pathol. 2023;45:238–52.
Aunkam P, Sibponkrung S, Limkul S, Seabkongseng T, Mahanil K, Umnajkitikorn K, et al. Mechanisms of cannabis growth promotion by Bacillus velezensis S141. Plants. 2024;13(21):2971.
Cavallero A, Chelucci E, Chiellini C, Gabriele M. Exploring microalgae and endophyte as biostimulants: antioxidant and anti-inflammatory properties of Cannabis sativa L. sprouts under standard and enrichment conditions. Food Biosci. 2024;62:105138.
Corredor-Perilla IC, Cuervo Andrade JL, Olejar KJ, Park SH. Beneficial properties of soil bacteria from Cannabis sativa L.: seed germination, phosphorus solubilization and mycelial growth inhibition of Fusarium sp. Rhizosphere. 2023;27,100780.
Lyu D, Backer R, Smith DL. Three plant growth-promoting rhizobacteria alter morphological development, physiology, and flower yield of Cannabis sativa L. Ind Crops Prod. 2022;178:114583.
Lyu D, Backer R, Berrué F, Martinez-Farina C, Hui JPM, Smith DL. Plant growth-promoting rhizobacteria (PGPR) with microbial growth broth improve biomass and secondary metabolite accumulation of Cannabis sativa L. J Agric Food Chem. 2023;71:7268–77.
Tanney CAS, Lyu D, Schwinghamer T, Geitmann A, Ruan ED, Smith DL. Sub-optimal nutrient regime coupled with Bacillus and Pseudomonas sp. inoculation influences trichome density and cannabinoid profiles in drug-type Cannabis sativa. Front Plant Sci. 2023;14,1131346.
Backer R, Schwinghamer T, Rosenbaum P, McCarty V, Eichhorn Bilodeau S, Lyu D, et al. Closing the yield gap for cannabis: a meta-analysis of factors determining cannabis yield. Front Plant Sci. 2019;10:495.
Lyu D, Backer R, Robinson WG, Smith DL. Plant growth-promoting rhizobacteria for cannabis production: yield, cannabinoid profile and disease resistance. Front Microbiol. 2019;10:461387.
Gonçalves J, Rosado T, Soares S, Simão AY, Caramelo D, Luís Â, et al. Cannabis and its secondary metabolites: their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines (Basel). 2019;6:31.
Punja ZK. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag Sci. 2021;77(9):3857–70.
Kusari P, Kusari S, Spiteller M, Kayser O. Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers. 2013;60:137–51.
Scott M, Rani M, Samsatly J, Charron J-B, Jabaji S. Endophytes of industrial hemp (Cannabis sativa L.) cultivars: identification of culturable bacteria and fungi in leaves, petioles, and seeds. Can J Microbiol. 2018;64:664–80.
Ruiz KB, Biondi S, Oses R, Acuña-Rodríguez IS, Antognoni F, Martinez-Mosqueira EA, et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron Sustain Dev. 2014;34:349–59.
Bazile D, Pulvento C, Verniau A, Al-Nusairi MS, Ba D, Breidy J, et al. Worldwide evaluations of quinoa: preliminary results from post international year of quinoa FAO projects in nine countries. Front Plant Sci. 2016;7:850.
Olmos E, Jiménez-Pérez B, Román-García I, Fernández-García N. Salt-tolerance mechanisms in quinoa: is glycinebetaine the missing piece of the puzzle? Plant Physiol Biochem. 2024;206:108276.
Maestro-Gaitán I, Granado-Rodríguez S, Poza-Viejo L, Matías J, Márquez-López JC, Pedroche JJ, et al. Quinoa plant architecture: a key factor determining plant productivity and seed quality under long-term drought. Environ Exp Bot. 2023;211:105350.
Yang A, Akhtar SS, Iqbal S, Amjad M, Naveed M, Zahir ZA, et al. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct Plant Biol. 2016;43:632–42.
Gonzales V, Huallpan M, Ramirez X, Miguel YS, Dubey M, Jensen DF, et al. Rhizosphere bacteria from the Bolivian highlands improve drought tolerance in quinoa (Chenopodium quinoa Willd.). J Appl Microbiol. 2024;135:lxae296.
Castiglione S, Oliva G, Vigliotta G, Novello G, Gamalero E, Lingua G, et al. Effects of compost amendment on glycophyte and halophyte crops grown on saline soils: Isolation and characterization of rhizobacteria with plant growth promoting features and high salt resistance. Appl Sci (Switzerland). 2021;11:1–15.
Mahdi I, Fahsi N, Hafidi M, Allaoui A, Biskri L. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis qa1 and Enterobacter asburiae qf11 isolated from Chenopodium quinoa willd. Microorganisms. 2020;8:1–21.
Mahdi I, Allaoui A, Fahsi N, Biskri L. Bacillus velezensis QA2 potentially induced salt stress tolerance and enhanced phosphate uptake in quinoa plants. Microorganisms. 2022;10:1836.
Li J, Guo X, Cai D, Xu Y, Wang Y. Bacillus amyloliquefaciens 11B91 inoculation enhances the growth of quinoa (Chenopodium quinoa Willd.) under salt stress. PeerJ. 2023;11:e15925.
Mahdi I, Fahsi N, Hafidi M, Benjelloun S, Allaoui A, Biskri L. Rhizospheric phosphate solubilizing Bacillus atrophaeus GQJK17 S8 increases quinoa seedling, withstands heavy metals, and mitigates salt stress. Sustainability. 2021;13(6):3307.
Slatni T, Ben Slimene I, Harzalli Z, Taamalli W, Smaoui A, Abdelly C, et al. Enhancing quinoa (Chenopodium quinoa) growth in saline environments through salt-tolerant rhizobacteria from halophyte biotope. Physiol Plant. 2024;176:e14466.
Rafique E, Mumtaz MZ, Ullah I, Rehman A, Qureshi KA, Kamran M, et al. Potential of mineral-solubilizing bacteria for physiology and growth promotion of Chenopodium quinoa Willd. Front Plant Sci. 2022;13:1004833.
Alvarado R, Fuentes A, Ortiz J, Herrera H, Arriagada C. Metal(loid)-resistant bacterial consortia with antimycotic properties increase tolerance of Chenopodium quinoa Willd. to metal(loid) stress. Rhizosphere. 2022;23:100569.
Alvarado R, Arriagada-Escamilla C, Ortiz J, Campos-Vargas R, Cornejo P. Alginate-bentonite encapsulation of extremophillic bacterial consortia enhances Chenopodium quinoa tolerance to metal stress. Microorganisms. 2024;12:2066.
Cai D, Xu Y, Zhao F, Zhang Y, Duan H, Guo X. Improved salt tolerance of Chenopodium quinoa Willd. contributed by Pseudomonas sp. strain M30–35. PeerJ. 2021;9:e10702.
Mahdi I, Hafidi M, Allaoui A, Biskri L. Halotolerant endophytic bacterium Serratia rubidaea ed1 enhances phosphate solubilization and promotes seed germination. Agriculture. 2021;11:224.
Aslam MU, Raza MAS, Saleem MF, Waqas M, Iqbal R, Ahmad S, et al. Improving strategic growth stage-based drought tolerance in Quinoa by rhizobacterial inoculation. Commun Soil Sci Plant Anal. 2020;51:853–68.
Valbuena-Rodríguez JL, Fonseca-Guerra I, Buitrago-Yomayusa C, Puentes-S A, Rozo MEB. Isolation and characterization of Pantoea ananatis and P. agglomerans in quinoa: P. ananatis as a potential fungal biocontroller and plant growth promoter. Int Microbiol. 2024;1–13.
Yang A, Akhtar SS, Fu Q, Naveed M, Iqbal S, Roitsch T, et al. Burkholderia phytofirmans PsJN stimulate growth and yield of Quinoa under salinity stress. Plants. 2020;9:672.
Khan A, Singh AV. Multifarious effect of ACC deaminase and EPS producing Pseudomonas sp. and Serratia marcescens to augment drought stress tolerance and nutrient status of wheat. World J Microbiol Biotechnol. 2021;37:198.
Abadi VAJM, Sepehri M, Rahmani HA, Zarei M, Ronaghi A, Taghavi SM, et al. Role of dominant phyllosphere bacteria with plant growth–promoting characteristics on growth and nutrition of maize (Zea mays L.). J Soil Sci Plant Nutr. 2020;20:2348–63.
Jaramillo Roman V, den Toom LA, Castro Gamiz C, van der Pijl N, Visser RGF, van Loo EN, et al. Differential responses to salt stress in ion dynamics, growth and seed yield of European quinoa varieties. Environ Exp Bot. 2020;177:104146.
Matías J, Rodríguez MJ, Cruz V, Calvo P, Granado-Rodríguez S, Poza-Viejo L, et al. Assessment of the changes in seed yield and nutritional quality of quinoa grown under rainfed Mediterranean environments. Front Plant Sci. 2023;14: 1268014.
Igiehon NO, Babalola OO, Aremu BR. Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019;19:159.
Maestro-Gaitan I, Redondo-Nieto M, Gonzalez-Bodi S, Maestro-Gaitán I, Redondo-Nieto M, González-Bodí S, et al. Insights into quinoa endophytes: core bacterial communities reveal high stability to water stress and genotypic variation. Environ Microbiome. 2025;20:16.
Maestro-Gaitán I, Granado-Rodríguez S, Redondo-Nieto M, Battaglia A, Poza-Viejo L, Matías J, et al. Unveiling changes in rhizosphere-associated bacteria linked to the genotype and water stress in quinoa. Microb Biotechnol. 2023;16:2326–44.
Fanai A, Bohia B, Lalremruati F, Lalhriatpuii N, Lalmuanpuii R, Singh PK. Plant growth promoting bacteria (PGPB)-induced plant adaptations to stresses: An updated review. PeerJ. 2024;12:e17882.
Singh A, Maurya A, Rajkumar S, Singh AK, Bhardwaj R, Kaushik SK, et al. Genome-wide comparative analysis of five amaranthaceae species reveals a large amount of repeat content. Plants. 2024;13: 824.
Nandan A, Koirala P, Dutt Tripathi A, Vikranta U, Shah K, Gupta AJ, et al. Nutritional and functional perspectives of pseudocereals. Food Chem. 2024;448:139072.
Netshimbupfe MH, Berner J, Van Der Kooy F, Oladimeji O, Gouws C. The importance and use of Amaranthus for crop diversification in the SADC region. S Afr J Bot. 2023;152:192–202.
Malik M, Sindhu R, Dhull SB, Bou-Mitri C, Singh Y, Panwar S, et al. Nutritional composition, functionality, and processing technologies for Amaranth. J Food Process Preserv. 2023;2023:1753029.
Bvenura C, Kambizi L. Future grain crops. Future foods: global trends, opportunities, and sustainability challenges. 2022;81–105.
Yadav A, Yadav K. From humble beginnings to nutritional powerhouse: the rise of Amaranth as a climate-resilient superfood. Trop Plants. 2020;0:1–15.
Devi R, Kaur T, Kour D, Yadav AN. Microbial consortium of mineral solubilizing and nitrogen fixing bacteria for plant growth promotion of amaranth (Amaranthus hypochondrius L.). Biocatal Agric Biotechnol. 2022;43:102404.
Pandey C, Dheeman S, Kumar Negi Y, Maheshwari K. Differential response of native Bacillus spp. isolates from agricultural and forest soils in growth promotion of Amaranthus hypochondriacus. Biotechnol Res. 2018;4:54–61.
Pandey C, Negi YK, Maheshwari DK, Rawat D, Prabha D. Potential of native cold tolerant plant growth promoting bacilli to enhance nutrient use efficiency and yield of Amaranthus hypochondriacus. Plant Soil. 2018;428:307–20.
Pandey C, Bajpai VK, Negi YK, Rather IA, Maheshwari DK. Effect of plant growth promoting Bacillus spp. on nutritional properties of Amaranthus hypochondriacus grains. Saudi J Biol Sci. 2018;25:1066–71.
Raj R, Johnson R, Joel JM, Nair SG, Cherian E, Job J, et al. Biopriming with a native microbial consortium favourably modulates the growth dynamics and yield of Amaranthus tricolor and Oryza sativa. J Plant Growth Regul. 2024;1–14.
Patel M, Vurukonda SSKP, Patel A. Multi-trait halotolerant plant growth-promoting bacteria mitigate induced salt stress and enhance growth of Amaranthus viridis. J Soil Sci Plant Nutr. 2023;23:1860–83.
Moreno-Espíndola IP, Ferrara-Guerrero MJ, De León-González F, Rivera-Becerril F, Mayorga-Reyes L, Pérez NO. Enzymatic activity and culturable bacteria diversity in rhizosphere of amaranth, as indicators of crop phenological changes. Bot Sci. 2018;96(4):640–9.
Parra-Cota FI, Peña-Cabriales JJ, De Los Santos-Villalobos S, Martínez-Gallardo NA, Délano-Frier JP. Burkholderia ambifaria and B. caribensis promote growth and increase yield in grain amaranth (Amaranthus cruentus and A. hypochondriacus) by improving plant nitrogen uptake. PLoS One. 2014;9:e88094.
Bhagyashree KB, Shivaprakash MK, Reddy MR. Isolation and identification of bacterial endophytes from grain Amaranth (Amaranthus caudatus) for plant growth promotion. Indian J Agric Res. 2023;57:426–30.
Sandhya S, Radhakrishnan R, Sathasivam R, Arun M, Packiaraj G, Park SU. Influence of endophytic bacterium, Cellulosimicrobium sp. FRR2 on plant growth of Amaranthus campestris L. and bacterial survival at adverse environmental conditions. J Pure Appl Microbiol. 2021;15:2288–94.
Yashaswini MS, Nysanth NS, Anith KN. Endospore-forming bacterial endophytes from Amaranthus spp. improve plant growth and suppress leaf blight (Rhizoctonia solani Kühn) disease of Amaranthus tricolor L. Rhizosphere. 2021;19:100387.
Radhakrishnan R, Ajithkumar P, Arun M, Sathasivam R, Sandhya S, Choi J, et al. An endophyte Paenibacillus dendritiformis strain APL3 promotes Amaranthus polygonoides L. sprout growth and their extract inhibits food-borne pathogens. Plant Sci Today. 2021;8:941–7.
Barba de la Rosa AP, Huerta-Ocampo JA, González-Escobar JL, Aguilar-Hernández HS, Salcedo-Barrientos G, Espitia-Rangel E. Differential expression of iron transporters in Amaranthus cruentus roots when are subjected to salt stress: The influence of root endophytes. Rhizosphere. 2022;24:100620.
Niharika K, Sheeba S. Effect of chromium species and plant growth promoting microorganisms on growth parameters of Amaranthus gangeticus. Int J Environ Clim Chang. 2022;1484–90.
Yuan M, He H, Xiao L, Zhong T, Liu H, Li S, et al. Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere. 2014;103:99–104.
Honrubia M, Andrino A, Morte A. Preparation and maintenance of both man-planted and wild plots. In: Kagan-Zur V, Roth-Bejerano N, Sitrit Y, Morte A, editors. Desert Truffles. Springer-Verlag: Berlin Heidelberg; 2014. p. 367–87.
Morte A, Honrubia M, Gutiérrez A. Biotechnology and cultivation of desert truffles. In: Varma A, editor. Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics (Third Edition). Springer-Verlag, Berlin: Heidelberg; 2008. p. 467–83.
Morte A, Andrino A. Domestication: preparation of mycorrhizal seedlings. In: Kagan-Zur V, Roth-Bejerano N, Sitrit Y, Morte A, editors. Desert truffles: phylogeny, physiology, distribution and domestication. Srpinger-Verlag Berlin Heidelberg; 2014. p. 343–65.
Navarro-Ródenas A, Berná LM, Lozano-Carrillo C, Andrino A, Morte A. Beneficial native bacteria improve survival and mycorrhization of desert truffle mycorrhizal plants in nursery conditions. Mycorrhiza. 2016;26:769–79.
Benucci GMN, Bonito GM. The truffle microbiome: species and geography effects on bacteria associated with fruiting bodies of hypogeous Pezizales. Microb Ecol. 2016;72:4–8.
Alhuthali S, Bello SK, Bageel AM, Shori AB, Bataweel NM, Al-Hejin AM, et al. Soil physicochemical and metagenomic analyses of bacteria and fungi: toward desert truffle cultivation in Saudi Arabia. Agronomy. 2024;14:3021.
Satish L, Barak H, Keren G, Yehezkel G, Kushmaro A, Ben-Dov E, et al. The microbiome structure of the symbiosis between the desert truffle Terfezia boudieri and its host plant Helianthemum sessiliflorum. J Fungi. 2022;8:1062.
Adeleke R, Dames JF. Kalaharituber pfeilii and associated bacterial interactions. S Afr J Bot. 2014;90:68–73.
Guarnizo Á L, Navarro-Ródenas A, Calvo-Polanco M, Marqués-Gálvez JE, Morte A. A mycorrhizal helper bacterium alleviates drought stress in mycorrhizal Helianthemum almeriense plants by regulating water relations and plant hormones. Environ Exp Bot. 2023;207:105228.
Sangwan S, Prasanna R. Mycorrhizae helper bacteria: unlocking their potential as bioenhancers of plant–arbuscular mycorrhizal fungal associations. Microb Ecol. 2022;84:1–10.
Frey-Klett P, Pierrat JC, Garbaye J. Location and survival of mycorrhiza helper Pseudomonas fluorescens during establishment of ectomycorrhizal symbiosis between Laccaria bicolor and Douglas fir. Appl Environ Microbiol. 1997;63:139–44.
Morte A, Navarro-Ródenas A, Nicolás E. Physiological parameters of desert truffle mycorrhizal Helianthemun almeriense plants cultivated in orchards under water deficit conditions. Symbiosis. 2010;52(2–3):133–9.
Navarro-Ródenas A, Bárzana G, Nicolás E, Carra A, Schubert A, Morte A. Expression analysis of aquaporins from desert truffle mycorrhizal symbiosis reveals a fine-tuned regulation under drought. Mol Plant Microbe Interact. 2013;26:1068–78.
Sharipova G, Ivanov R, Veselov D, Akhiyarova G, Shishova M, Nuzhnaya T, et al. Involvement of Reactive Oxygen Species in ABA-Induced Increase in Hydraulic Conductivity and Aquaporin Abundance. Int J Mol Sci. 2021;22:9144.
Charpentier M, Sun J, Wen J, Mysore KS, Oldroyd GED. Abscisic acid promotion of arbuscular mycorrhizal colonization requires a component of the protein phosphatase 2A complex. Plant Physiol. 2014;166:2077–90.
Hill RA, Wong ‐ Bajracharya J, Anwar S, Coles D, Wang M, Lipzen A, et al. Abscisic acid supports colonization of Eucalyptus grandis roots by the mutualistic ectomycorrhizal fungus Pisolithus microcarpus. New Phytol. 2022;233:966–82.
Martín – Rodríguez JA, León – Morcillo R, Vierheilig H, Ocampo JA, Ludwig – Müller J, García – Garrido JM. Ethylene – dependent/ethylene – independent ABA regulation of tomato plants colonized by arbuscular mycorrhiza fungi. New Phytol. 2011;190:193–205.
Zhang F, Wang P, Zou Y-N, Wu Q-S, Kuča K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch Agron Soil Sci. 2019;65:1316–30.
Benjamin G, Pandharikar G, Frendo P. Salicylic acid in plant symbioses: beyond plant pathogen interactions. Biology. 2022;11:861.
Herrera Medina M. Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Sci. 2003;164:993–8.
Marqués-Gálvez JE, Morte A, Navarro-Ródenas A. Spring stomatal response to vapor pressure deficit as a marker for desert truffle fruiting. Mycorrhiza. 2020;30:503–12.
Arenas F, López-García Á, Berná LM, Morte A, Navarro-Ródenas A. Desert truffle mycorrhizosphere harbors organic acid releasing plant growth–promoting rhizobacteria, essentially during the truffle fruiting season. Mycorrhiza. 2022;32:193–202.
Adnan M, Shah Z, Fahad S, Arif M, Alam M, Khan IA, et al. Phosphate-solubilizing bacteria nullify the antagonistic effect of soil calcification on bioavailability of phosphorus in alkaline soils. Sci Rep. 2017;7:16131.
Etesami H, Adl SM. Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. Phyto-Microbiome in stress regulation. 2020;147–203.
Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaat EVL, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013;64:807–38.
Cordovez V, Dini-Andreote F, Carrión VJ, Raaijmakers JM. Ecology and evolution of plant microbiomes. Annu Rev Microbiol. 2019;73:69–88.
Zachow C, Müller H, Tilcher R, Berg G. Differences between the rhizosphere microbiome of Beta vulgaris ssp. maritima-ancestor of all beet crops-and modern sugar beets. Front Microbiol. 2014;5:70954.
Sasse J, Martinoia E, Northen T. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 2018;23:25–41.
Tomasi N, Weisskopf L, Renella G, Landi L, Pinton R, Varanini Z, et al. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol Biochem. 2008;40:1971–4.