Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta. 2010;1802(4):416–31.
Kandilis AN, Papadopoulou IP, Koskinas J, Sotiropoulos G, Tiniakos DG. Liver innervation and hepatic function: new insights. J Surg Res. 2015;194(2):511–9.
Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(1):827–35.
Ahren B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia. 2000;43(4):393–410.
Hampton RF, Jimenez-Gonzalez M, Stanley SA. Unravelling innervation of pancreatic islets. Diabetologia. 2022;65(7):1069–84.
Taborsky GJ Jr, Mundinger TO. Minireview: The role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology. 2012;153(3):1055–62.
Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–79.
McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am. 2012;41(1):57–87.
Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6(3):1239–78.
Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30(8):1433–40.
Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–65.
Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22(5):565–604.
Miller BM, Oderberg IM, Goessling W. Hepatic nervous system in development, regeneration, and disease. Hepatology. 2021;74(6):3513–22.
Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(1):808–20.
Fukuda Y, Imoto M, Koyama Y, Miyazawa Y, Hayakawa T. Demonstration of noradrenaline-immunoreactive nerve fibres in the liver. J Int Med Res. 1996;24(6):466–72.
Tsuneki K, Ichihara K. Electron microscope study of vertebrate liver innervation. Arch Histol Jpn. 1981;44(1):1–13.
Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8(3):169–77.
Ramkissoon R, Gardner TB. Pancreatic steatosis: an emerging clinical entity. Am J Gastroenterol. 2019;114(11):1726–34.
Tang SC, Baeyens L, Shen CN, Peng SJ, Chien HJ, Scheel DW, et al. Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia. 2018;61(1):168–81.
Rebours V, Gaujoux S, d’Assignies G, Sauvanet A, Ruszniewski P, Levy P, et al. Obesity and fatty pancreatic infiltration are risk factors for pancreatic precancerous lesions (PanIN). Clin Cancer Res. 2015;21(15):3522–8.
Schaefer PM, Kalinina S, Rueck A, von Arnim CAF, von Einem B. NADH autofluorescence-a marker on its way to boost bioenergetic research. Cytometry A. 2019;95(1):34–46.
Wallrabe H, Svindrych Z, Alam SR, Siller KH, Wang T, Kashatus D, et al. Segmented cell analyses to measure redox states of autofluorescent NAD(P)H, FAD & Trp in cancer cells by FLIM. Sci Rep. 2018;8(1):79.
Croce AC, Ferrigno A, Bottiroli G, Vairetti M. Autofluorescence-based optical biopsy: an effective diagnostic tool in hepatology. Liver Int. 2018;38(7):1160–74.
Croce AC, Ferrigno A, Santin G, Vairetti M, Bottiroli G. Bilirubin: an autofluorescence bile biomarker for liver functionality monitoring. J Biophotonics. 2014;7(10):810–7.
Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47(6):719–30.
Di Guardo G. Lipofuscin, lipofuscin-like pigments and autofluorescence. Eur J Histochem. 2015;59(1):2485.
Ueda Y, Kobayashi M. Spectroscopic studies of autofluorescence substances existing in human tissue: influences of lactic acid and porphyrins. Appl Opt. 2004;43(20):3993–8.
Shrirao AB, Schloss RS, Fritz Z, Shrirao MV, Rosen R, Yarmush ML. Autofluorescence of blood and its application in biomedical and clinical research. Biotechnol Bioeng. 2021;118(12):4550–76.
Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM). J Histochem Cytochem. 2001;49(12):1565–72.
Erben T, Ossig R, Naim HY, Schnekenburger J. What to do with high autofluorescence background in pancreatic tissues – an efficient Sudan black B quenching method for specific immunofluorescence labelling. Histopathology. 2016;69(3):406–22.
Chien HJ, Chiang TC, Peng SJ, Chung MH, Chou YH, Lee CY, et al. Human pancreatic afferent and efferent nerves: mapping and 3-D illustration of exocrine, endocrine, and adipose innervation. Am J Physiol Gastrointest Liver Physiol. 2019;317(5):G694–706.
Chung MH, Chien HJ, Peng SJ, Chou YH, Chiang TC, Chang HP, et al. Multimodal 3-D/2-D human islet and duct imaging in exocrine and endocrine lesion environment: associated pancreas tissue remodeling. Am J Physiol Endocrinol Metab. 2022;323(4):E354–65.
Sakr N, Glazova O, Shevkova L, Onyanov N, Kaziakhmedova S, Shilova A, et al. Characterizing and quenching autofluorescence in fixed mouse adrenal cortex tissue. Int J Mol Sci. 2023;24(4):3432.
Zhang Z, Fan H, Richardson W, Gao BZ, Ye T. Management of autofluorescence in formaldehyde-fixed myocardium: choosing the right treatment. Eur J Histochem. 2023;67(4):3812.
Oliveira VC, Carrara RC, Simoes DL, Saggioro FP, Carlotti CG Jr, Covas DT, et al. Sudan Black B treatment reduces autofluorescence and improves resolution of in situ hybridization specific fluorescent signals of brain sections. Histol Histopathol. 2010;25(8):1017–24.
Sun Y, Yu H, Zheng D, Cao Q, Wang Y, Harris D. Sudan black B reduces autofluorescence in murine renal tissue. Arch Pathol Lab Med. 2011;135(10):1335–42.
Radtke AJ, Chu CJ, Yaniv Z, Yao L, Marr J, Beuschel RT, et al. IBEX: an iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues. Nat Protoc. 2022;17(2):378–401.
Liu CH, Lin CH, Tsai MJ, Chen WT, Chai CY, Huang YC, et al. Melanin bleaching with dilute hydrogen peroxide: a simple and rapid method. Appl Immunohistochem Mol Morphol. 2013;21(3):275–9.
Chung JY, Choi J, Sears JD, Ylaya K, Perry C, Choi CH, et al. A melanin-bleaching methodology for molecular and histopathological analysis of formalin-fixed paraffin-embedded tissue. Lab Invest. 2016;96(10):1116–27.
Duong H, Han M. A multispectral LED array for the reduction of background autofluorescence in brain tissue. J Neurosci Methods. 2013;220(1):46–54.
Nolta NF, Liberti A, Makol R, Han M. Gelatin embedding and LED autofluorescence reduction for rodent spinal cord histology. J Neurosci Methods. 2020;1(346):108924.
Zheng J, Wu YC, Phillips EH, Cai X, Wang X, Seung-Young Lee S. Increased multiplexity in optical tissue clearing-based three-dimensional immunofluorescence microscopy of the tumor microenvironment by light-emitting diode photobleaching. Lab Invest. 2024;104(6):102072.
Ueda HR, Erturk A, Chung K, Gradinaru V, Chedotal A, Tomancak P, et al. Tissue clearing and its applications in neuroscience. Nat Rev Neurosci. 2020;21(2):61–79.
Richardson DS, Guan W, Matsumoto K, Pan C, Chung K, Erturk A, et al. Tissue clearing. Nat Rev Methods Primers. 2021. https://doi.org/10.1038/s43586-021-00080-9.
Tainaka K, Kuno A, Kubota SI, Murakami T, Ueda HR. Chemical principles in tissue clearing and staining protocols for whole-body cell profiling. Annu Rev Cell Dev Biol. 2016;6(32):713–41.
Holtzer RL, Van Lancker JL. Early changes in pancreas autolysis. Am J Pathol. 1962;40(3):331–6.
Shimizu M, Hayashi T, Saitoh Y, Ohta K, Itoh H. Postmortem autolysis in the pancreas: multivariate statistical study. The influence of clinicopathological conditions. Pancreas. 1990;5(1):91–4.
Walker AE. The adult pancreas in trauma and disease. Acad Forensic Pathol. 2018;8(2):192–218.
Alwelaie Y, Point du Jour KS, Pandya S, Goodman AL, Centeno BA, Adsay V, et al. Acinar cell induced autolysis is a frequent occurrence in CytoLyt-fixed pancreatic fine needle aspiration specimens: an analysis of 157 cytology samples. Cancer Cytopathol. 2021;129(4):283–90.
Qadir MMF, Alvarez-Cubela S, Weitz J, Panzer JK, Klein D, Moreno-Hernandez Y, et al. Long-term culture of human pancreatic slices as a model to study real-time islet regeneration. Nat Commun. 2020;11(1):3265.
Alver CG, Alvarez-Cubela S, Altilio I, Hutchison E, Warrner E, Viso ME, et al. SliceChip: a benchtop fluidic platform for organotypic culture and serial assessment of human and rodent pancreatic slices. Lab Chip. 2024;24(6):1557–72.
Monici M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev. 2005;11:227–56.
DiMaio VJ, DiMaio D. Forensic pathology. 2nd ed. Boca Raton: CRC Press; 2001.
Demchenko AP. Photobleaching of organic fluorophores: quantitative characterization, mechanisms, protection. Methods Appl Fluoresc. 2020;8(2):022001.
Bernas T, Zarebski M, Dobrucki JW, Cook PR. Minimizing photobleaching during confocal microscopy of fluorescent probes bound to chromatin: role of anoxia and photon flux. J Microsc. 2004;215(Pt 3):281–96.
Kwon J, Elgawish MS, Shim SH. Bleaching-resistant super-resolution fluorescence microscopy. Adv Sci (Weinh). 2022;9(9):e2101817.
Patterson GH, Piston DW. Photobleaching in two-photon excitation microscopy. Biophys J. 2000;78(4):2159–62.
Dasgupta A, Koerfer A, Kokot B, Urbancic I, Eggeling C, Carravilla P. Effects and avoidance of photoconversion-induced artifacts in confocal and STED microscopy. Nat Methods. 2024;21(7):1171–4.
Haugland RP. The handbook: a guide to fluorescent probes and labeling technologies. 10th ed. Waltham: Invitrogen Corp; 2005.
Aitken CE, Marshall RA, Puglisi JD. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys J. 2008;94(5):1826–35.
Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305(5686):1007–9.
Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 2008;322(5904):1065–9.
Power RM, Huisken J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat Methods. 2017;14(4):360–73.
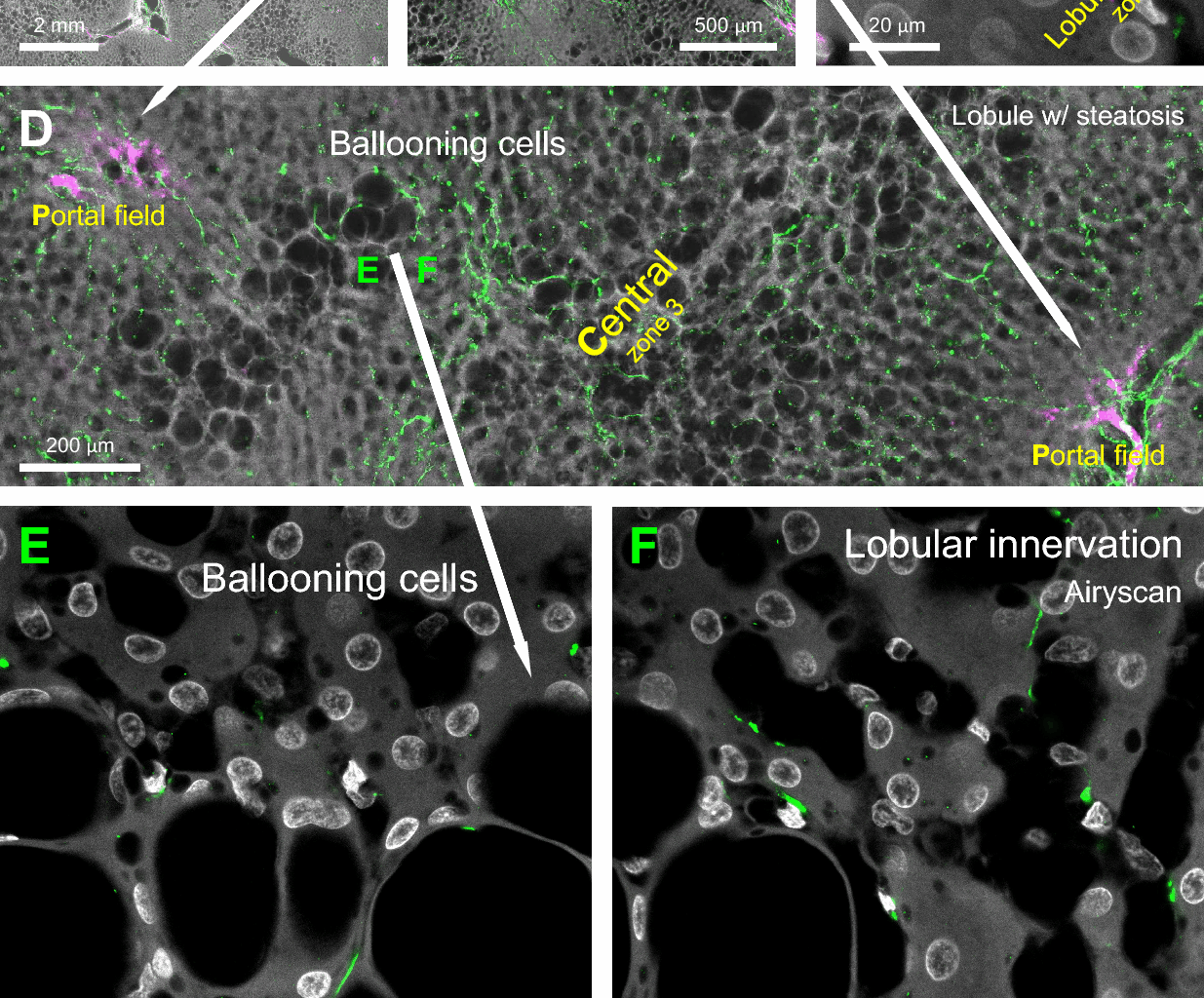
Adori C, Daraio T, Kuiper R, Barde S, Horvathova L, Yoshitake T, et al. Disorganization and degeneration of liver sympathetic innervations in nonalcoholic fatty liver disease revealed by 3D imaging. Sci Adv. 2021. https://doi.org/10.1126/sciadv.abg5733.
Liu K, Yang L, Wang G, Liu J, Zhao X, Wang Y, et al. Metabolic stress drives sympathetic neuropathy within the liver. Cell Metab. 2021;33(3):666-75 e4.
Durgam S, Singh B, Cole SL, Brokken MT, Stewart M. Quantitative assessment of tendon hierarchical structure by combined second harmonic generation and immunofluorescence microscopy. Tissue Eng Part C Methods. 2020;26(5):253–62.
Chapman KB, Filipsky F, Peschke N, Gelleri M, Weinhardt V, Braun A, et al. A comprehensive method to study the DNA’s association with Lamin And Chromatin compaction in intact cell nuclei at super resolution. Nanoscale. 2023;15(2):742–56.
Florijn RJ, Slats J, Tanke HJ, Raap AK. Analysis of antifading reagents for fluorescence microscopy. Cytometry. 1995;19(2):177–82.
Arsic A, Stajkovic N, Spiegel R, Nikic-Spiegel I. Effect of Vectashield-induced fluorescence quenching on conventional and super-resolution microscopy. Sci Rep. 2020;10(1):6441.
Hsiao FT, Chien HJ, Chou YH, Peng SJ, Chung MH, Huang TH, et al. Transparent tissue in solid state for solvent-free and antifade 3D imaging. Nat Commun. 2023;14(1):3395.
Chen CC, Peng SJ, Chou YH, Lee CY, Lee PH, Hu RH, et al. Human liver afferent and efferent nerves revealed by 3-D/Airyscan super-resolution imaging. Am J Physiol Endocrinol Metab. 2024;326(2):E107–23.
Lee CY, Kuo TC, Chou YH, Peng SJ, Hsiao FT, Chung MH, et al. 3D imaging resolves human pancreatic duct-beta-cell clusters during cystic change. Diabetes. 2025;74(5):734–48.
ZEISS Lattice SIM 5. Resolve the details hiding in the depth. https://www.zeiss.com/microscopy/us/products/light-microscopes/super-resolution-microscopes/lattice-sim-5.html. Accessed 30 September 2025.
Nikon Instruments Inc. Application note: precision imaging in complex tissue Structures. https://www.microscope.healthcare.nikon.com/resources/application-notes/precision-imaging-in-complex-tissue-structures. Accessed 30 September 2025.
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–112.
Burt AD, Mutton A, Day CP. Diagnosis and interpretation of steatosis and steatohepatitis. Semin Diagn Pathol. 1998;15(4):246–58.
Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–46.
Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat Rev Endocrinol. 2012;8(12):743–54.
Tien YW, Chien HJ, Chiang TC, Chung MH, Lee CY, Peng SJ, et al. Local islet remodelling associated with duct lesion-islet complex in adult human pancreas. Diabetologia. 2021;64(10):2266–78.
Dodt HU, Leischner U, Schierloh A, Jahrling N, Mauch CP, Deininger K, et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods. 2007;4(4):331–6.
Erturk A, Becker K, Jahrling N, Mauch CP, Hojer CD, Egen JG, et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc. 2012;7(11):1983–95.
Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of neural science. 5th ed. Columbus: McGraw-Hill; 2013.
Usdin TB, Eiden LE, Bonner TI, Erickson JD. Molecular biology of the vesicular ACh transporter. Trends Neurosci. 1995;18(5):218–24.
Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378(4):454–67.
Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84(2):361–76.
Amenta F, Cavallotti C, Ferrante F, Tonelli F. Cholinergic nerves in the human liver. Histochem J. 1981;13(3):419–24.
Akiyoshi H, Gonda T, Terada T. A comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig, dog and human livers. Liver. 1998;18(5):352–9.
Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol (Berl). 1992;186(5):431–42.
Berthoud HR, Powley TL. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of Dil. Brain Res. 1991;553(2):336–41.
Hwang J, Okada J, Liu L, Pessin JE, Schwartz GJ, Jo YH. The development of hepatic steatosis depends on the presence of liver-innervating parasympathetic cholinergic neurons in mice fed a high-fat diet. PLoS Biol. 2024;22(10):e3002865.
Metz CN, Pavlov VA. Vagus nerve cholinergic circuitry to the liver and the gastrointestinal tract in the neuroimmune communicatome. Am J Physiol Gastrointest Liver Physiol. 2018;315(5):G651–8.
Berthoud HR, Munzberg H, Morrison CD, Neuhuber WL. Hepatic interoception in health and disease. Auton Neurosci. 2024;253:103174.
Berthoud HR, Fox EA, Munzberg H, Yu S, Kim A, Lowell BB, et al. Direct vagal input to the gastrointestinal tract and other viscera: Re-definition of autonomic neuroscience or experimental artifacts? Auton Neurosci. 2025;260:103310.
Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286–94.
Li ZS, Fox-Threlkeld JE, Furness JB. Innervation of intestinal arteries by axons with immunoreactivity for the vesicular acetylcholine transporter (VAChT). J Anat. 1998;192(Pt 1):107–17.
Tang SC, Shen CN, Lin PY, Peng SJ, Chien HJ, Chou YH, et al. Pancreatic neuro-insular network in young mice revealed by 3d panoramic histology. Diabetologia. 2018;61(1):158–67.
Huff J. The Airyscan detector from ZEISS: confocal imaging with improved signal-to-noise ratio and super-resolution. Nature Methods. 2015 Dec;12(12).
Begg DP, Woods SC. Interactions between the central nervous system and pancreatic islet secretions: a historical perspective. Adv Physiol Educ. 2013;37(1):53–60.
Lkhagvasuren B, Mee-Inta O, Zhao ZW, Hiramoto T, Boldbaatar D, Kuo YM. Pancreas-brain crosstalk. Front Neuroanat. 2021;15:691777.
Rosario W, Singh I, Wautlet A, Patterson C, Flak J, Becker TC, et al. The brain-to-pancreatic islet neuronal map reveals differential glucose regulation from distinct hypothalamic regions. Diabetes. 2016;65(9):2711–23.
Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34(2):206–13.
Teff KL. Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans. Physiol Behav. 2010;99(3):317–23.
Smeets PA, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutr Rev. 2010;68(11):643–55.
Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50(5):1030–8.
Ahren B. Islet nerves in focus–defining their neurobiological and clinical role. Diabetologia. 2012;55(12):3152–4.
Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21.
Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127(12):4217–27.
Drucker DJ. GLP-1-based therapies for diabetes, obesity and beyond. Nat Rev Drug Discov. 2025.
Booth MJ. Adaptive optics in microscopy. Philos Trans A Math Phys Eng Sci. 1861;2007(365):2829–43.
Wassie AT, Zhao Y, Boyden ES. Expansion microscopy: principles and uses in biological research. Nat Methods. 2019;16(1):33–41.
Bykov YS, Cortese M, Briggs JA, Bartenschlager R. Correlative light and electron microscopy methods for the study of virus-cell interactions. FEBS Lett. 2016;590(13):1877–95.