Microorganism
Yeast isolates, isolated from wounds, were obtained from the microbiological laboratory at Tanta University, Egypt, and were cultured on Sabouraud dextrose agar (SDA). The identification of C. albicans isolates was performed using CHROMagar™ Candida medium, Gram-staining, germ tube test, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). For the preparation of inoculum, C. albicans was cultured on SDA, incubated at 37 °C for 48 h before each experiment, and was standardized using 0.5 McFarland with a final concentration of approximately 106 colony-forming units per milliliter (CFU/ml) [19,20,21].
Antifungal Susceptibility Testing (AFST) for C. albicans and detection of MDR isolates
The AFST was performed on Mueller-Hinton agar supplemented with 2% glucose (2%) and methylene blue (0.00005%) using the Kirby-Bauer disk diffusion method according to CLSI (2009). The examined antifungal agents were miconazole (MIC 30 µg), amphotericin-B (AMB 100 µg), nystatin (NS 50 IU), fluconazole (FLC 10 µg), and itraconazole (IT 10 µg) [7]. After that, the MDR isolates were identified according to Arendrup & Patterson (2017), who stated that the Candida isolates are defined as MDR if they are nonsusceptible to 1 or more antifungal agents in 2 or more antifungal classes [22]. Candida albicans (MTCC 227) was used as a standard strain.
Antifungal activity of β-carotene against C. albicans using agar well diffusion assay
The antifungal activity was performed by agar well diffusion method against the isolated C. albicans, as El Zawawy et al. prescribed [23]. Firstly, the SDA with 8-mm wells was inoculated with 100 µl of each C. albicans strain (0.5 McFarland). Then, a volume of 100 µl of β-carotene (200 µg/ml), purchased from Sigma-Aldrich and prepared by dissolving the powder in 10% DMSO, was added to one well, and the other well was filled with 10% DMSO as a negative control. After 24 h of incubation at 37 °C, the results were detected, and the diameter of the inhibition zone around each well was measured. (Amphotericin-B was used as a positive control).
Minimum Inhibitory Concentration (MIC) determination via broth microdilution assay
The MIC of β-carotene against C. albicans isolates was tested by broth microdilution assay, as detailed by Sun et al. [24]. In a 96-well microtiter, a serial dilution of β-carotene was prepared from a stock (20 mg/ml), starting from 100 µg/ml and ending with 3.125 µg/ml using Sabouraud dextrose broth (SDB). The concentration of prepared C. albicans suspension was adjusted to 4 × 103 CFU/ml by SDB, and 100 µl of C. albicans suspension was placed into wells filled with 100 µl of SDB, which contained β-carotene, and fungal growth or inhibition was observed after incubation at 37 °C for 24 h. At the first well in each column, C. albicans suspension in SDB (200 µl) acted as a growth control, and un-inoculated SDB (200 µl) was added to the last well as a negative control. Finally, the MIC was determined as the lowest concentration at which no visual growth was observed, and the experiments were repeated in triplicate. Amphotericin-B was used as a positive control.
Analysis of growth kinetics using time-kill assay against MDR isolates
The anticandidal activity was assessed with 0, 0.25, 0.5, 1, and 2 MICs of β-carotene against MDR C. albicans (CA 2, 3, 5, 6, 8, 14, 17, and 20) and C. albicans MTCC 227, and the resultant growth profile for 12 h was plotted as detailed by Rajeh et al. [25]. β-Carotene was prepared in desired concentrations in a vial containing 15 ml SDB, and then the cell suspension was adjusted to an optical density (OD) of 0.15 at 600 nm, and 10% DMSO-containing medium acted as a control. Then, the vials were incubated at 37 °C for 12 h, and a volume of 1 ml was taken from each vial to measure OD at 600 nm every 2 h. Finally, the growth profile was plotted using Microsoft Excel. (The experiments were repeated in triplicate).
Biofilm formation ability of C. albicans using the Crystal Violet (CV) technique
According to Anggraini et al. the CV technique was used to assess the ability of C. albicans isolates to generate biofilms [26]. A volume of 200 µl of the prepared yeast suspension (106 cells/ml) in SDB, supplemented with 1% glucose, was inoculated in a flat-bottom 96-well microplate, and then the plate was incubated at 37 °C under static conditions to allow biofilm formation. Following a 24-h incubation period, the extra media was discarded, and the wells were air-dried after being cleaned three times with 200 µl of phosphate-buffered saline (PBS) to get rid of any detached cells using a micropipette. Following that, 150 µl of methanol was added for 20 min to enhance biofilm attachment, and the plate was left to air dry. Then 180 µl of 0.1% CV was applied to each well for 15 min. After discarding the CV, the residual stain was eliminated with three rounds of washing with sterile distilled water (SDW). Finally, a microtiter reader (SunriseTM, TECAN, Switzerland) was used to measure the OD at 595 nm after adding 180 µl of 33% (vol/vol) glacial acetic acid to dissolve the embedded dye within biofilms. Uninoculated SDB were used as negative controls, and each strain of C. albicans filled up three wells. C. albicans (MTCC 227) was used as a standard strain. The resulting biofilms were classified according to the cut-off value (OD value of the negative control (ODC)). (The experiments were repeated in triplicate).
Biofilm is negative when OD ≤ ODC.
Biofilm is slightly positive when ODC ≤ OD ≤ 2 x ODC.
Biofilm is moderately positive when 2 × ODC ≤ OD ≤ 4 × ODC.
Biofilm is strongly positive when 4 × ODC ≤ OD.
Antibiofilm activity of β-carotene against strong biofilm-forming C. albicans using microtiter plate and silicon catheters assays
The effect of β-carotene on the mature biofilms of C. albicans (CA 2, 3, 5, 6, 8, 14, 17, 20, and MTCC 227) was evaluated, as prescribed by Feldman et al. [27]. At first, the biofilm was established as mentioned above, and the wells were washed twice with PBS, and then a volume of 200 µl of fresh SDB containing β-carotene (100 and 200 µg/ml) was added. The SDB containing 10% DMSO served as a control. After the plate was incubated for 24 h at 37 °C, the biofilms were measured using the 1% CV assay as stated above, and the assay was performed in triplicate by measuring OD at 595 nm. The percentage of eradication in the biofilm was calculated by using the following formula;
$$\begin{aligned} &\mathrm{Percentage}\;\mathrm{of}\;\mathrm{biofilm}\;\mathrm{eradication}\\&=\;\frac{\mathrm{OD}\left(\mathrm{untreated}\right)\;-\;\mathrm{OD}\left(\mathrm{treated}\right)}{\mathrm{OD}\;\left(\mathrm{untreated}\right)}\;\times100\end{aligned}$$
$$\begin{aligned} \mathrm{OD}\;(\mathrm{untreated})\;=\;&\mathrm{OD}\;\mathrm{at}\;595\;\mathrm{nm}\;\mathrm{of}\;\\&\mathrm{untreated}\mathit\;C\mathit.\mathit\;albicans.\end{aligned}$$
$$\begin{aligned} \mathrm{OD}\;\left(\mathrm{treated}\right)\;=\;&\mathrm{OD}\;\mathrm{at}\;595\;\mathrm{nm}\;\mathrm{of}\;\mathrm\beta-\mathrm{carotene}\\&-\mathrm{treated}\;\mathrm{or}\;10\%\;\mathrm{DMSO}\\&-\mathrm{treated}\;C\mathit.\mathit\;albicans.\end{aligned}$$
Furthermore, the antibiofilm ability of β-carotene was emphasized using silicon catheters, as described by Wongchai et al. with a few modifications [28]. Silicon catheter pieces with a 50 mm incision were immersed in a 6-well microplate containing 5 ml of yeast culture in SDB supplemented with 1% glucose (1 × 106 cells/ml). After that, the plate was incubated under static conditions at 37 °C to enable the formation of biofilms on silicon catheters. The wells were washed twice with PBS, and a volume of 5 ml of fresh SDB containing either β-carotene (100 and 200 µg/ml) or 10% DMSO (control) was added. Following a 24-h incubation period at 37 °C, the wells were rinsed with 0.85% NaCl, the catheter segments were stained for 15 min using a 0.1% CV, rinsed with 0.85% NaCl, and resolved in 95% ethanol. The OD at 595 nm was then used to determine the biofilm eradication utilizing the aforementioned formula. The statistical analysis was performed by comparing the percentage of biofilm eradication after treatment of the C. albicans (untreated) biofilms with β-carotene (100 and 200 µg/ml) with the percentage of biofilm eradication after treatment the C. albicans biofilms with the negative control (10% DMSO). (Amphotericin-B was used as a positive control).
Light Microscopy (LM) to evaluate the effect of β-carotene on biofilm, adherence, and hyphae formation of C. albicans
As described by Venkatramanan et al. the effect of β-carotene on the biomass of C. albicans biofilms (CA 2, 3, 5, 6, 8, 14, 17, 20, and MTCC 227) employing CV staining was detected utilizing the light microscopic examination method [29]. In brief, a 6-well microtitre plate with glass coverslips was filled with 2 ml of the prepared C. albicans suspension in SDB + 1% glucose, and the plate was then incubated at 37 °C in a static state. Following the incubation period of 24 h at 37 °C, the wells were twice rinsed with PBS before being filled with 2 ml of fresh SDB, both with β-carotene (100 and 200 µg/ml) and with 10% DMSO as a control. After 24-h incubation at 37 °C, the media were removed from treated and untreated wells to remove planktonic cells, and the glass coverslips were rinsed with SDW and then stained with 0.1% CV. Lastly, after two rounds of washing of excessive CV with SDW and air drying, the adherent biofilms on glass coverslips were seen under a LM at 1000-x. Furthermore, the antibiofilm activity of β-carotene was confirmed via dual staining technique using Maneval’s stain and Congo red, per the instructions provided by Manhas et al. [30]. Following the establishment of the β-carotene-treated and 10% DMSO-treated C. albicans biofilms as previously mentioned, the biofilms were washed with SDW, and then the biofilms were fixed for 30 min at room temperature using formaldehyde solution (4%). After air-drying, the fixed biofilms were dyed with 1% Congo red stain and allowed to dry, and then stained with Maneval’s stain for 10 min. After that, the coverslips were cleaned by gentle washing with SDW and dried at room temperature. Finally, the biofilms were inspected under a LM at 1000-x magnification. In case of LM examination of the effect of β-carotene on adherence, a 6-well microtitre plate with glass coverslips was filled with 2 ml of the prepared C. albicans suspension in SDB + 1% glucose, both with β-carotene (0.25 and 0.5 MICs) and with 10% DMSO as a control, and the plate was incubated in a static condition at 37 °C. Then, the media was removed from the wells to eliminate the planktonic cells after a 24-h incubation period at 37 °C, and the glass coverslips were rinsed with SDW and stained with 0.1% CV. After the removal of excessive stain and air-drying, the adhering cells on glass coverslips were finally observed under a LM [31]. In case of hyphae detection, the impact of β-carotene on yeast-to-hyphal phase transition was investigated using LM, as detailed by Sun et al. [24]. An overnight cultured C. albicans was diluted (2 × 106 CFU/ml) in RPMI-1640 medium + 15% fetal bovine serum (FBS), both with β-carotene (0.25 and 0.5 MICs) and 10% DMSO as a control. After 4 h of incubation at 37 °C with constant shaking at 160 rpm, the phase transition in treated and untreated cells was visualized using a LM at 1000-x magnification. (Amphotericin-B was used as a positive control).
Confocal Laser Scanning Microscopy (CLSM) of biofilm cells
According to Sun et al. the CLSM evaluated the impact of β-carotene on the pre-established biofilms of C. albicans (CA 14) [32]. Briefly, the β-carotene (100 and 200 µg/ml)-treated and 10% DMSO-treated biofilms were established in an 8-well chamber slide, as mentioned above, where 10% DMSO acted as a negative control. Following the three times washing with PBS to remove non-adherent cells, the biofilm was co-stained for 15 min in the dark using 30 µl of each of propidium iodide (PI) and acridine orange (AO), and the treated and untreated biofilms were screened by CLSM (DMi8; Leica Microsystem). The biofilm was fully visualized in three dimensions using Z-stacks, and the fluorescence intensity was ascertained using ImageJ’s histogram. (Amphotericin-B was used as a positive control).
Impact of β-carotene on the adherence ability of C. albicans on polystyrene plates
The effect of β-carotene on the adherence of C. albicans (CA 2, 3, 5, 6, 8, 14, 17, 20, and MTCC 227) was inspected via CV staining using 96-well and 6-well microtitre plates as prescribed by Awadelkareem et al. [31]. The ultimate concentration of 5 × 106 cells/ml was achieved by adjusting an overnight C. albicans culture in SDB medium (supplemented with 1% glucose), containing either β-carotene (0.25 and 0.5 MICs) or 10% DMSO as a control. A 96-well polystyrene plate with a flat bottom was filled with 200 µl of C. albicans cell suspensions, and the plates were kept at 37 °C for 24 h. After that, the planktonic cells were discarded, and 200 µl of PBS was used to gently wash the wells. Following that, adherent cells were stained with 0.1% CV (200 µl), and further incubation was carried out for 30 min at 37 °C. The excess dye was carefully removed using PBS solution, microtiter plates were fixed with 95% ethanol for 15 min at 37 °C, and the sample’s absorbance was measured with a spectrophotometer at 595 nm. To determine the percentage inhibition, the formula [(OD (control) − OD (test)/OD (control)] × 100 was used. C. albicans (MTCC 227) was used as a standard strain, and amphotericin-B was used as a positive control.
Viability of planktonic and sessile cells
The SDB was used to adjust an overnight C. albicans culture to a final concentration of 5 × 106 cells/ml. The culture was then incubated for 3 h at 37 °C with agitation, either with β-carotene (200 µg/ml)-containing SDB or with 10% DMSO-containing SDB as a control. Following their centrifugation, the cell pellets were resuspended in 20 µl of SDB. Next, 2 µl of a 0.1% methylene blue solution was combined with 5 µl of the cell suspension on a slide glass, and a cover slip was placed over the mixture. A LM (LABOMED, CXL, USA) was used to view the cell morphology and viability at a magnification of 1000-x [33]. In case of viability detection of sessile cells, as previously reported by Thieme et al. the number of live fungal cells was counted in the presence and absence of β-carotene (100 and 200 µg/ml) in order to ascertain the impact of β-carotene on the viability of sessile cells of C. albicans within pre-established biofilms [34]. In summary, following the above-mentioned biofilm formation of C. albicans (CA 2, 3, 5, 6, 8, 14, 17, 20, and MTCC 227) using the microtiter plate method, both with β-carotene and with 10% DMSO as a control, the microtiter plate wells were twice washed with PBS to remove any loosely attached cells. After using a pipette tip to scrape the biofilms out of the wells, 200 µl of PBS was added to each well, and the biofilms were then homogenized using a vortex. After the biofilm was homogenized, it was serially diluted with PBS. About 1 ml of each dilution was plated onto a petri dish, and then the molten SDA was poured to count the colony-forming unit (CFU) after a 48-h incubation period at 37 °C. Furthermore, on a slide glass, a volume of 5 µl of the homogenized and diluted biofilms, both treated and untreated, was combined with 2 µl of a 0.1% methylene blue solution, and a cover slip was positioned on top of the mixture. The cell viability was examined using a LM at a 1000-x magnification [33]. (Amphotericin-B was used as a positive control).
Effect of β-carotene on polysaccharide matrix using phenol sulfuric acid assay
The impact of β-carotene on exopolysaccharide (EPS) was evaluated in accordance with El Zawawy et al. [23]. A 6-well microplate was filled with 2 ml of a prepared C. albicans culture in SDB + 1% glucose (106 cells/ml), containing both β-carotene (0.25 and 0.5 MICs) or 10% DMSO as a negative control. The non-adherent cells in the treated and untreated biofilms were removed after a 24-h incubation period at 37 °C. A volume of 2 ml of 0.9% NaCl was then added to the wells, and they were thoroughly cleaned. Following that, sterile test tubes containing 1 ml of 5% phenol were filled with 1 ml of cell suspensions in 0.9% NaCl. After adding 5 ml of 96% sulphuric acid and letting it stand in the dark for 1 h, a UV-Vis spectrophotometer was used to detect the absorbance at 490 nm (Genesys™ 10 S, Thermo Scientific, USA). C. albicans (MTCC 227) was used as a standard strain, and amphotericin-B was used as a positive control. The percentage of reduction in EPS production was calculated by using the following formula;
$$\begin{aligned} &\mathrm{Percentage}\;\mathrm{of}\;\mathrm{reduction}\;\mathrm{in}\;\mathrm{EPS}\;\mathrm{production}\;\\&=\;\frac{\mathrm{OD}\left(\mathrm{untreated}\right)\;-\;\mathrm{OD}\left(\mathrm{treated}\right)}{\mathrm{OD}\;\left(\mathrm{untreated}\right)}\;\times100\end{aligned}$$
$$\mathrm{OD}\;(\mathrm{untreated})\;=\;\mathrm{OD}\;\mathrm{at}\;490\;\mathrm{nm}\;\mathrm{of}\;\mathrm{untreated}\mathit\;C\mathit.\mathit\;albicans.$$
$$\begin{aligned} \mathrm{OD}\;(\mathrm{treated})\;=\;&\mathrm{OD}\;\mathrm{at}\;490\;\mathrm{nm}\;\mathrm\beta-\mathrm{carotene}\\&-\mathrm{treated}\;\mathrm{or}\;10\%\;\mathrm{DMSO}\\&-\mathrm{treated}\;C\mathit.\mathit\;albicans. \end{aligned}$$
The statistical analysis was performed by comparing the percentage of reduction in EPS production by C. albicans after treatment with β-carotene (0.25 and 0.5 MICs) with the percentage of reduction of EPS by C. albicans after treating with 10% DMSO.
Effect of β-carotene on hyphae-specific gene by quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
The influence of β-carotene on the expression of the hypha-specific gene, ALS3, was assessed by qRT-PCR. C. albicans suspension in RPMI-1640 medium (106 CFU/ml) was treated with 0.5 MIC of β-carotene under shaking conditions for 4 h at 37 °C, where 10% DMSO served as a control. The fungal cells were washed, and the total RNA was extracted employing TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and the cDNA was synthesized via TransScript® First-Strand cDNA Synthesis SuperMix (TransGen, Beijing, China). Primers for the ALS3 gene and the housekeeping internal control gene, the ACT1 gene, were listed in Table S1. The following parameters were used for qRT-PCR using Rotor-Gene Q (Qiagen, USA): initial denaturation (3 min/95°C), followed by 40 cycles of denaturation (1 min/95°C), annealing (30 s/58°C), and extension (20 s/72°C). The CT value of the ALS3 gene was normalized to the CT value of the ACT1 gene, and the relative gene expression was determined using the 2−ΔΔCT assay [35]. (Amphotericin-B was used as a positive control)
Detecting the interaction of β-carotene with agglutinin-like protein 3 (Als3) using molecular docking analysis
The crystallographic structure of the Als3 protein (PDB: 4LEE) was retrieved from the Protein Data Bank (https://www.rcsb.org/structure/4LEE), and the β-carotene structure was downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/compound/5280489). After energy minimization and preparation of Als3 protein and β-carotene via the Autodock Tools (ADT) software, the AutoDock Vina program was utilized to execute molecular docking to assess the binding affinities of β-carotene with Als3 protein [36, 37].
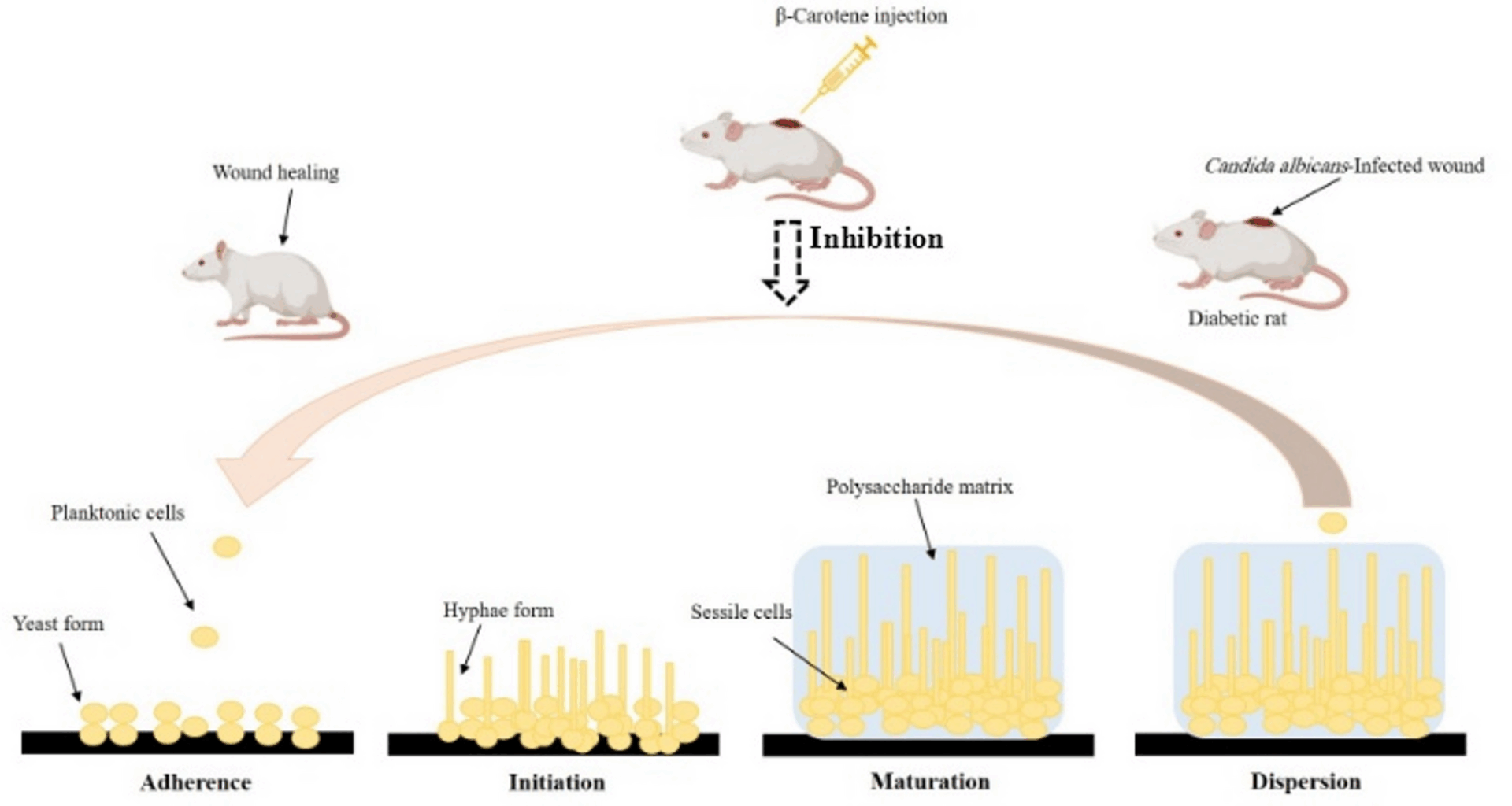
Assessment of anticandidal activity of β-carotene against MDR C. albicans-infected diabetic wounds in a rat model
Anticandidal action of β-carotene against MDR C. albicans was studied using a wound infection model in diabetic rats, and the Research Ethics Committee of the Faculty of Pharmacy at Tanta University (TP/RE/2/25p-02) allowed the model steps in accordance with ARRIVE guidelines [6, 38].
Step 1: A total of 20 male Sprague–Dawley rats, aged between 10 and 12 weeks, weighing between 300 and 400 g, were obtained from the animal house of the Faculty of Pharmacy at Tanta University (Tanta, Egypt), and the rats were categorized into 2 groups (control and treated).
Step 2: Rats were kept separately in vented cages with 12 h of light and 12 h of darkness, at room temperature, and with unhindered access to food and drink to adjust to their surroundings.
Step 3: Diabetes was produced in rats after 7 days of acclimatization with an intraperitoneal injection of streptozocin (STZ) (Sigma-Aldrich) at a dosage of 50 mg/kg for two consecutive days.
Step 4: Following 48 h of the second injection, the blood glucose level was measured after being drawn from the tail vein, and the rats were identified as diabetic when their glucose level exceeded 200 mg/dL.
Step 5: Rats that had been anesthetized with ketamine (40 mg/kg) and xylazine (5 mg/kg) were shaved from the back side and sterilized with 10% povidone-iodine. After that, the excisional wound (10 mm thick) was created on the rat’s dorsal portion using biopsy punches.
Step 6: The MDR C. albicans (CA 14) cell suspension (5 × 107 CFU/ml) was subcutaneously injected (100 µl) into the diabetic wounds for two consecutive days.
Step 7: On the next day, the control group was subcutaneously administered 20 µl of the vehicle (10% DMSO as a negative control), and β-carotene (200 µg/ml) was subcutaneously injected into the treated group.
Step 8: A sterile ruler was used to determine the wound size at day 0, 4, 8, and 12, and the wound healing ratios were calculated using the formula below.
$$\begin{aligned} &\mathrm{Wound}\;\mathrm{healing}\;\mathrm{ratio}\;\\&=\frac{\mathrm{Initial}\;\mathrm{wound}\;\mathrm{size}\;-\mathrm{the}\;\mathrm{current}\;\mathrm{wound}\;\mathrm{size}\;\mathrm{measured}\;\mathrm{by}\;\mathrm{ruler}}{\mathrm{Initial}\;\mathrm{wound}\;\mathrm{size}}\;\\&\times\;100 \end{aligned}$$
Step 9: On day 12 of the experiment, rats from each group were put to death by CO2 inhalation, and the skin lesions (2 to 5 mm) were removed.
Step 10: After the wound samples were collected, they were preserved in a 10% buffered formalin solution, embedded in a paraffin block, sectioned at a thickness of 5 m, and stained with hematoxylin and eosin (H & E) for histological examination.
Step 11: Tissues were homogenized using a sonicator, and the resulting suspensions were serially diluted and plated on SDA to determine the fungal count. The following formula was used to determine the tissue’s fungal burden:
$$\begin{aligned} \mathrm{CFU}/\mathrm g=\left(\mathrm{plate}\;\mathrm{count}\times\frac1{dilution}\times10\right)/\mathrm{weight}\;\mathrm{of}\;\mathrm{homogenized}\;\mathrm{tissue} \end{aligned}$$
Statistical analysis
The data were reported as mean ± SD, and all experiments were run in triplicate, except for AFST. GraphPad Prism version 5 software was utilized to compare the two groups using a t-test. The p-value < 0.05 served as a significance level.