Ke H, Tang S, Guo T, Hou D, Jiao X, Li S, et al. Landscape of pathogenic mutations in premature ovarian insufficiency. Nat Med. 2023;29(2):483–92. https://doi.org/10.1038/s41591-022-02194-3.
Rahman R, Panay N. Diagnosis and management of premature ovarian insufficiency. Best Pract Res Clin Endocrinol Metab. 2021;35(6):101600. https://doi.org/10.1016/j.beem.2021.101600.
Federici S, Rossetti R, Moleri S, Munari EV, Frixou M, Bonomi M, Persani L. Primary ovarian insufficiency: update on clinical and genetic findings. Front Endocrinol (Lausanne). 2024;15:1464803. https://doi.org/10.3389/fendo.2024.1464803.
Hagen-Lillevik S, Johnson J, Lai K. Early postnatal alterations in follicular stress response and survival in a mouse model of classic galactosemia. J Ovarian Res. 2022;15(1):122. https://doi.org/10.1186/s13048-022-01049-2.
Nash Z, Davies M. Premature ovarian insufficiency. BMJ. 2024;384:e077469. https://doi.org/10.1136/bmj-2023-077469.
Liu Y, Pan Z, Wu Y, Song J, Chen J. Comparison of anti-Müllerian hormone and antral follicle count in the prediction of ovarian response: a systematic review and meta-analysis. J Ovarian Res. 2023;16(1):117. https://doi.org/10.1186/s13048-023-01202-5.
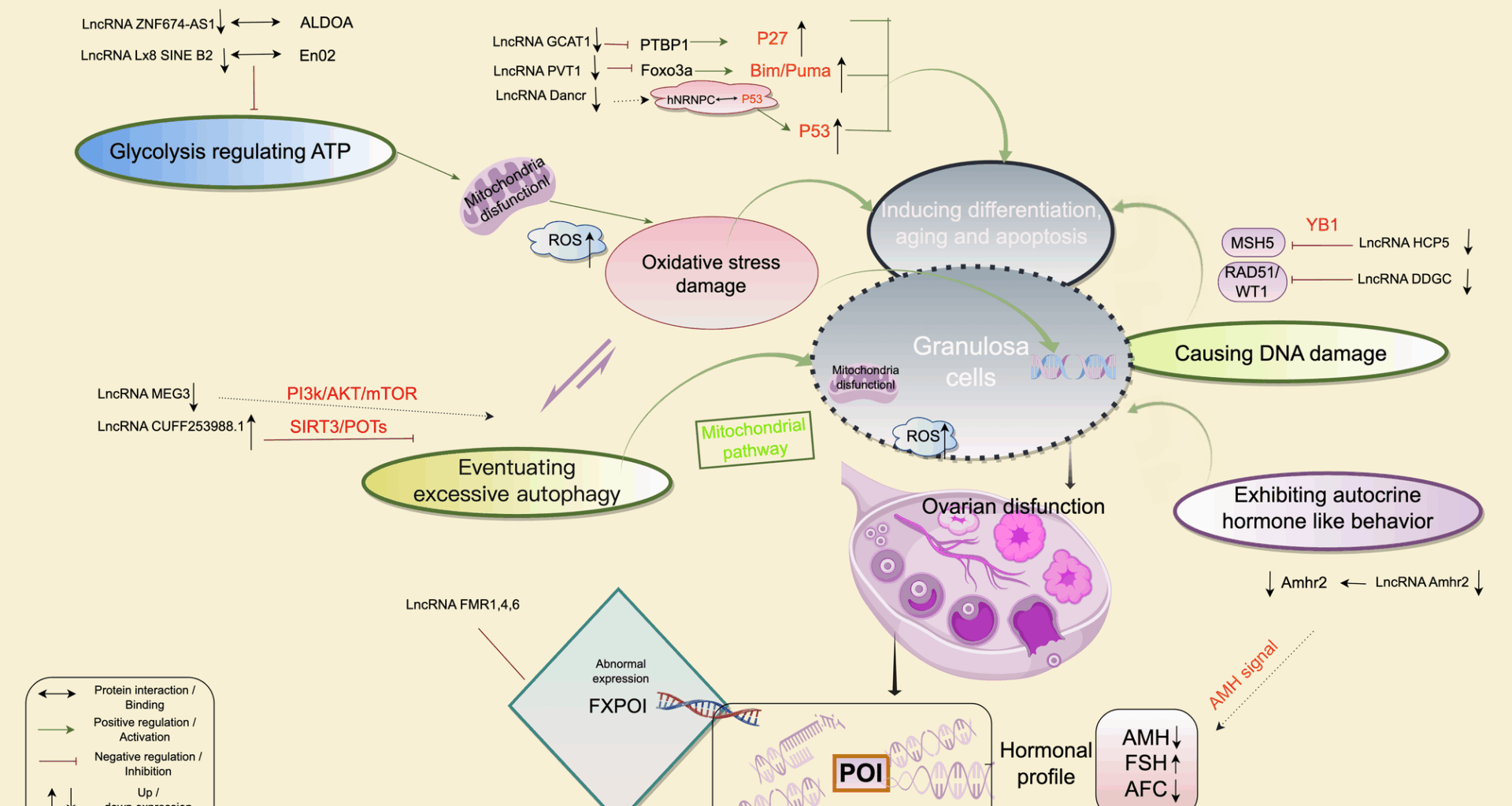
Zhang JH, Chen JH, Guo B, Fang Y, Xu ZY, Zhan L, Cao YX. Recent insights into noncoding RNAs in primary ovarian insufficiency: focus on mechanisms and treatments. J Clin Endocrinol Metab. 2023;108(8):1898–908. https://doi.org/10.1210/clinem/dgad070.
Binder AK, Bremm F, Dörrie J, Schaft N. Non-coding RNA in tumor cells and tumor-associated myeloid cells: function and therapeutic potential. Int J Mol Sci. 2024;25(13):7275. https://doi.org/10.3390/ijms25137275.
Bibi A, Bartekova M, Gandhi S, Greco S, Madè A, Sarkar M, et al. Circular RNA regulatory role in pathological cardiac remodelling. Br J Pharmacol. 2025;182(2):316–39. https://doi.org/10.1111/bph.16434.
Bagheri M, Khansarinejad B, Mondanizadeh M, Azimi M, Alavi S. MiRNAs related in signaling pathways of women’s reproductive diseases: an overview. Mol Biol Rep. 2024;51(1):414. https://doi.org/10.1007/s11033-024-09357-0.
Dragomir M, Chen B, Calin GA. Exosomal LncRNAs as new players in cell-to-cell communication. Transl Cancer Res. 2018;7(Suppl 2):S243–52. https://doi.org/10.21037/tcr.2017.10.46.
Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297–1325. https://doi.org/10.1152/physrev.00041.2015.
Mohan N, Dashwood RH, Rajendran P. A–Z of epigenetic readers: targeting alternative splicing and histone modification variants in cancer. Cancers (Basel). 2024;16(6):1104. https://doi.org/10.3390/cancers16061104.
Zhu Q, Sun J, An C, Li X, Xu S, He Y, et al. Mechanism of LncRNA Gm2044 in germ cell development. Front Cell Dev Biol. 2024;12:1410914. https://doi.org/10.3389/fcell.2024.1410914.
Kimura AP, Yoneda R, Kurihara M, Mayama S, Matsubara S. A long noncoding RNA, lncRNA-Amhr2, plays a role in Amhr2 gene activation in mouse ovarian granulosa cells. Endocrinology. 2017;158(11):4105–21. https://doi.org/10.1210/en.2017-00619.
Wang F, Chen X, Sun B, Ma Y, Niu W, Zhai J, Sun Y. Hypermethylation-mediated downregulation of LncRNA PVT1 promotes granulosa cell apoptosis in premature ovarian insufficiency via interacting with Foxo3a. J Cell Physiol. 2021;236(7):5162–75. https://doi.org/10.1002/jcp.30222.
Wang X, Zhang X, Dang Y, Li D, Lu G, Chan WY, et al. Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res. 2020;48(8):4480–91. https://doi.org/10.1093/nar/gkaa127.
Li D, Wang X, Li G, Dang Y, Zhao S, Qin Y. LncRNA ZNF674-AS1 regulates granulosa cell Glycolysis and proliferation by interacting with ALDOA. Cell Death Discov. 2021;7(1):493. https://doi.org/10.1038/s41420-021-00493-1.
Cho SH, Kim JH, Park HW, Park HS, An HJ, Kim YR, et al. Associations between HOTAIR polymorphisms and risk of primary ovarian insufficiency in Korean women. Maturitas. 2021;144:74–80. https://doi.org/10.1016/j.maturitas.2020.10.023.
Li D, Wang X, Dang Y, Zhang X, Zhao S, Lu G, et al. LncRNA GCAT1 is involved in premature ovarian insufficiency by regulating p27 translation in granulosa cells via competitive binding to PTBP1. Mol Ther Nucleic Acids. 2020;21:41–52. https://doi.org/10.1016/j.omtn.2020.10.041.
Li D, Xu W, Wang X, Dang Y, Xu L, Lu G, et al. LncRNA DDGC participates in premature ovarian insufficiency through regulating RAD51 and WT1. Mol Ther Nucleic Acids. 2021;26:15–27. https://doi.org/10.1016/j.omtn.2021.10.015.
Sun D, Wang Y, Sun N, Jiang Z, Li Z, Wang L, et al. LncRNA DANCR counteracts premature ovarian insufficiency by regulating the senescence process of granulosa cells through stabilizing the interaction between p53 and hNRNPC. J Ovarian Res. 2023;16(1):115. https://doi.org/10.1186/s13048-023-01115-3.
Ma X, Xu R, Chen J, Wang S, Hu P, Wu Y, et al. The epithelial Na (+) channel in ovarian granulosa cells modulates Ca(2+) mobilization and gonadotrophin signaling for Estrogen homeostasis and female fertility. Cell Commun Signal. 2024;22(1):398. https://doi.org/10.1186/s12964-024-01778-5.
Zanjirband M, Baharlooie M, Safaeinejad Z, Nasr-Esfahani MH. Transcriptomic screening to identify hub genes and drug signatures for PCOS based on RNA-Seq data in granulosa cells. Comput Biol Med. 2023;154:106601. https://doi.org/10.1016/j.compbiomed.2023.106601.
Han Y, Diao J, Wang X, Zhang S, Yuan L, Ping Y, et al. Single-cell RNA sequencing reveals common interactions between follicle immune cells and granulosa cells in premature ovarian insufficiency patients. Biol Reprod. 2025;112(1):156–68. https://doi.org/10.1093/biolre/ioae157.
Tu J, Chen Y, Li Z, Yang H, Chen H, Yu Z. Long non-coding RNAs in ovarian granulosa cells. J Ovarian Res. 2020;13(1):63. https://doi.org/10.1186/s13048-020-00663-2.
Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, et al. Induced NcRNAs allosterically modify RNA-binding proteins in Cis to inhibit transcription. Nature. 2008;454(7200):126–30. https://doi.org/10.1038/nature06992.
Luo C, Wei L, Qian F, Bo L, Gao S, Yang G, Mao C. LncRNA HOTAIR regulates autophagy and proliferation mechanisms in premature ovarian insufficiency through the miR-148b-3p/ATG14 axis. Cell Death Discov. 2024;10(1):44. https://doi.org/10.1038/s41420-024-01811-z.
Rižner TL, Romano A. Targeting the formation of estrogens for treatment of hormone-dependent diseases: current status. Front Pharmacol. 2023;14:1155558. https://doi.org/10.3389/fphar.2023.1155558.
Fatica A, Bozzoni I. Long non-coding rnas: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. https://doi.org/10.1038/nrg3606.
Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97(1):17–27. https://doi.org/10.1016/s0092-8674(00)80711-4.
Anderson RA, Cameron D, Clatot F, Demeestere I, Lambertini M, Nelson SM, Peccatori F. Anti-Müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. Hum Reprod Update. 2022;28(3):417–34. https://doi.org/10.1093/humupd/dmac004.
Panay N, Anderson RA, Bennie A, Cedars M, Davies M, Ee C, et al. Evidence-based guideline: premature ovarian insufficiency. Hum Reprod Open. 2024;2024(4):hoae065. https://doi.org/10.1093/hropen/hoae065.
Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22(6):709–24. https://doi.org/10.1093/humupd/dmw027.
Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and Cardiolipin in aging. Free Radic Biol Med. 2010;48(10):1286–95. https://doi.org/10.1016/j.freeradbiomed.2010.02.020.
Wen X, Tang L, Zhong R, Liu L, Chen L, Zhang H. Role of mitophagy in regulating intestinal oxidative damage. Antioxid (Basel). 2023;12(2):480. https://doi.org/10.3390/antiox12020480.
Tiosano D, Mears JA, Buchner DA, et al. Mitochondrial dysfunction in primary ovarian insufficiency. Endocrinology. 2019;160(10):2353–66. https://doi.org/10.1210/en.2019-00441.
Yang F, Zou Y. The effect of LncRNA MEG3 regulation of AMPK-mTOR autophagy pathway on H₂O₂-induced oxidative stress damage in human ovarian granulosa cells. Chin J Eugen Genet. 2024;32(7):1327–35. (in Chinese).
Chen X, Tang H, Liang Y, Wu P, Xie L, Ding Y, et al. Acupuncture regulates the autophagy of ovarian granulosa cells in polycystic ovarian syndrome ovulation disorder by inhibiting the PI3K/AKT/mTOR pathway through LncMEG3. Biomed Pharmacother. 2021;144:112288. https://doi.org/10.1016/j.biopha.2021.112288.
Chen Y, Chen Y, Cui X, He Q, Li H. Down-regulation of MALAT1 aggravates polycystic ovary syndrome by regulating miR-302d-3p-mediated leukemia inhibitory factor activity. Life Sci. 2021;277:119076. https://doi.org/10.1016/j.lfs.2021.119076.
Yang C, Fan H, Wu Y, Liang Z, Wang Y, Wu A, et al. T-2 toxin exposure induces ovarian damage in sows: LncRNA CUFF.253988.1 promotes cell apoptosis by inhibiting the SIRT3/PGC1α pathway. Ecotoxicol Environ Saf. 2024;283:116787. https://doi.org/10.1016/j.ecoenv.2024.116787.
Zhao Y, Zhou L, Li H, Sun T, Wen X, Li X, et al. Nuclear-encoded LncRNA MALAT1 epigenetically controls metabolic reprogramming in HCC cells through the mitophagy pathway. Mol Ther Nucleic Acids. 2021;23:264–76. https://doi.org/10.1016/j.omtn.2020.09.040.
Shi C, Zhang L, Qin C. Long non-coding RNAs in brain development, synaptic biology, and alzheimer’s disease. Brain Res Bull. 2017;132:160–9. https://doi.org/10.1016/j.brainresbull.2017.03.010.
Xu S, Herschman HR. A tumor agnostic therapeutic strategy for hexokinase 1-null/hexokinase 2-positive cancers. Cancer Res. 2019;79(23):5907–14. https://doi.org/10.1158/0008-5472.can-19-1789.
Watanuki S, Kobayashi H, Sugiura Y, Yamamoto M, Karigane D, Shiroshita K, et al. SDHAF1 confers metabolic resilience to aging hematopoietic stem cells by promoting mitochondrial ATP production. Cell Stem Cell. 2024;31(8):1145–e116115. https://doi.org/10.1016/j.stem.2024.04.023.
Chen F, Li X, Feng X, Gao T, Zhang W, Cheng Z, et al. Long noncoding RNA Lx8-SINE B2 interacts with Eno1 to regulate self-renewal and metabolism of embryonic stem cells. Stem Cells. 2022;40(12):1094–106. https://doi.org/10.1093/stmcls/sxac067.
Sun Z, Zhu M, Lv P, Cheng L, Wang Q, Tian P, et al. The long noncoding RNA Lncenc1 maintains naïve States of mouse ESCs by promoting the Glycolysis pathway. Stem Cell Rep. 2018;11(3):741–55. https://doi.org/10.1016/j.stemcr.2018.08.001.
Kalamara V, Garinis GA. The epitranscriptome: reshaping the DNA damage response. Trends Cell Biol. 2024;34(9):727–38. https://doi.org/10.1016/j.tcb.2024.06.008.
Leem J, Lee C, Choi DY, Oh JS. Distinct characteristics of the DNA damage response in mammalian oocytes. Exp Mol Med. 2024;56(2):319–28. https://doi.org/10.1038/s12276-024-01178-2.
Zhou FY, Waterman DP, Ashton M, Caban-Penix S, Memisoglu G, Eapen VV, Haber JE. Prolonged cell cycle arrest in response to DNA damage in yeast requires the maintenance of DNA damage signaling and the spindle assembly checkpoint. eLife. 2024;13:e94334. https://doi.org/10.7554/eLife.94334.
Maiuri T, Suart CE, Hung CLK, Graham KJ, Barba Bazan CA, Truant R. DNA damage repair in huntington’s disease and other neurodegenerative diseases. Neurotherapeutics. 2019;16(4):948–56. https://doi.org/10.1007/s13311-019-00768-7.
Colacurci N, De Leo V, Ruvolo G, Piomboni P, Caprio F, Pivonello R, et al. Recombinant FSH improves sperm DNA damage in male infertility: a phase II clinical trial. Front Endocrinol (Lausanne). 2018;9:383. https://doi.org/10.3389/fendo.2018.00383.
Morio T. Recent advances in the study of immunodeficiency and DNA damage response. Int J Hematol. 2017;106(3):357–65. https://doi.org/10.1007/s12185-017-2263-8.
Luo Z, Huang Y, Chen S, Zhang B, Huang H, Dabiri S, et al. Delivery of PARP inhibitors through 2HG-incorporated liposomes for synergistically targeting DNA repair in cancer. Cancer Lett. 2024;604:217268. https://doi.org/10.1016/j.canlet.2024.217268.
Wu H, Han Y, Liu J, Zhao R, Dai S, Guo Y, et al. The assembly and activation of the PANoptosome promote Porcine granulosa cell programmed cell death during follicular Atresia. J Anim Sci Biotechnol. 2024;15(1):147. https://doi.org/10.1186/s40104-024-01107-3.
Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, et al. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkeys. J Assist Reprod Genet. 2015;32(7):1069–78. https://doi.org/10.1007/s10815-015-0483-5.
Ma LZ, Wang A, Lai YH, Zhang J, Zhang XF, Chen SL, Zhou XY. USP14 Inhibition promotes DNA damage repair and represses ovarian granulosa cell senescence in premature ovarian insufficiency. J Transl Med. 2024;22(1):834. https://doi.org/10.1186/s12967-024-05636-3.
Dou X, Guo T, Li G, Zhou L, Qin Y, Chen ZJ. Minichromosome maintenance complex component 8 mutations cause primary ovarian insufficiency. Fertil Steril. 2016;106(6):1485–e14922. https://doi.org/10.1016/j.fertnstert.2016.08.018.
Guo T, Zhao S, Zhao S, Chen M, Li G, Jiao X, et al. Mutations in MSH5 in primary ovarian insufficiency. Hum Mol Genet. 2017;26(8):1452–7. https://doi.org/10.1093/hmg/ddx044.
Weinberg-Shukron A, Rachmiel M, Renbaum P, Gulsuner S, Walsh T, Lobel O, et al. Essential role of BRCA2 in ovarian development and function. N Engl J Med. 2018;379(11):1042–9. https://doi.org/10.1056/NEJMoa1800024.
Qin Y, Zhang F, Chen ZJ. BRCA2 in ovarian development and function. N Engl J Med. 2019;380(11):1086. https://doi.org/10.1056/NEJMc1813800.
Liu B, Liu L, Sulaiman Z, Wang C, Wang L, Zhu J, et al. Comprehensive analysis of lncRNA–miRNA–mRNA CeRNA network and key genes in granulosa cells of patients with biochemical primary ovarian insufficiency. J Assist Reprod Genet. 2023;40(10):2047–58. https://doi.org/10.1007/s10815-023-02937-2.
Huang QY, Chen SR, Chen JM, Shi QY, Lin S. Therapeutic options for premature ovarian insufficiency: an updated review. Reprod Biol Endocrinol. 2022;20(1):28. https://doi.org/10.1186/s12958-022-00892-8.
Rosario R, Stewart HL, Choudhury NR, Michlewski G, Charlet-Berguerand N, Anderson RA. Evidence for a fragile X messenger ribonucleoprotein 1 (FMR1) mRNA gain-of-function toxicity mechanism contributing to the pathogenesis of fragile X-associated premature ovarian insufficiency. FASEB J. 2022;36(11):e22612. https://doi.org/10.1096/fj.202200468RR.
Murray A, Ennis S, MacSwiney F, Webb J, Morton NE. Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet. 2000;8(4):247–52. https://doi.org/10.1038/sj.ejhg.5200451.
Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29(4):299–307. https://doi.org/10.1055/s-0031-1280915.
Huang J, Zhang W, Liu Y, Liu Y, Wang J, Jiang H. Association between the FMR1 CGG repeat lengths and the severity of idiopathic primary ovarian insufficiency: a meta-analysis. Artif Cells Nanomed Biotechnol. 2019;47(1):3116–22. https://doi.org/10.1080/21691401.2019.1645153.
Ma L, Herren AW, Espinal G, Randol J, McLaughlin B, Martinez-Cerdeño V, et al. Composition of the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome. Acta Neuropathol Commun. 2019;7(1):143. https://doi.org/10.1186/s40478-019-0796-1.
Bianchi E, Barbagallo F, Valeri C, Geremia R, Salustri A, De Felici M, Sette C. Ablation of the Sam68 gene impairs female fertility and gonadotropin-dependent follicle development. Hum Mol Genet. 2010;19(24):4886–94. https://doi.org/10.1093/hmg/ddq422.
Pagano G, Lyakhovich A, Pallardó FV, Tiano L, Zatterale A, Trifuoggi M. Mitochondrial dysfunction in fragile X syndrome and fragile X-associated tremor/ataxia syndrome: prospective use of antioxidants and mitochondrial nutrients. Mol Biol Rep. 2024;51(1):480. https://doi.org/10.1007/s11033-024-09415-7.
Alvarez-Mora MI, Rodriguez-Revenga L, Madrigal I, Guitart-Mampel M, Garrabou G, Milà M. Impaired mitochondrial function and dynamics in the pathogenesis of FXTAS. Mol Neurobiol. 2017;54(9):6896–902. https://doi.org/10.1007/s12035-016-0194-7.
Khalil AM, Faghihi MA, Modarresi F, Brothers SP, Wahlestedt C. A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS ONE. 2008;3(1):e1486. https://doi.org/10.1371/journal.pone.0001486.
Pastori C, Peschansky VJ, Barbouth D, Mehta A, Silva JP, Wahlestedt C. Comprehensive analysis of the transcriptional landscape of the human FMR1 gene reveals two new long noncoding RNAs differentially expressed in fragile X syndrome and fragile X-associated tremor/ataxia syndrome. Hum Genet. 2014;133(1):59–67. https://doi.org/10.1007/s00439-013-1356-6.
Elizur SE, Dratviman-Storobinsky O, Derech-Haim S, Lebovitz O, Dor J, Orvieto R, Cohen Y. FMR6 May play a role in the pathogenesis of fragile X-associated premature ovarian insufficiency. Gynecol Endocrinol. 2016;32(4):334–7. https://doi.org/10.3109/09513590.2015.1116508.
Allen EG, Charen K, Hipp HS, Shubeck L, Amin A, He W, et al. Refining the risk for fragile X-associated primary ovarian insufficiency by FMR1 CGG repeat size. Genet Med. 2021;23(9):1648–55. https://doi.org/10.1038/s41436-021-01177-y.
Tu J, Chen Y, Li Z, Yang H, Chen H, Yu Z. Long non-coding RNAs in ovarian granulosa cells. J Ovarian Res. 2020;13(1):63. https://doi.org/10.1186/s13048-020-00663-2.
Hu H, Zhang J, Xin X, Jin Y, Zhu Y, Zhang H, et al. Bushen Jianpi Tiaoxue Decoction inhibits the LIF–mTOR signaling axis to regulate mitochondrial function and alleviate cyclophosphamide-induced diminished ovarian reserve. Apoptosis. 2025;30(5–6):1331–50. https://doi.org/10.1007/s10495-025-02093-1.
Klinge CM. Estrogenic control of mitochondrial function. Redox Biol. 2020;31:101435. https://doi.org/10.1016/j.redox.2020.101435.
Huang J, Zhao J, Geng X, Chu W, Li S, Chen ZJ, Du Y. Long non-coding RNA lnc-CCNL1-3:1 promotes granulosa cell apoptosis and suppresses glucose uptake in women with polycystic ovary syndrome. Mol Ther Nucleic Acids. 2021;23:614–28. https://doi.org/10.1016/j.omtn.2020.12.008.
Jin L, Yang Q, Zhou C, Liu L, Wang H, Hou M, et al. Profiles for long non-coding RNAs in ovarian granulosa cells from women with PCOS with or without hyperandrogenism. Reprod Biomed Online. 2018;37(5):613–23. https://doi.org/10.1016/j.rbmo.2018.08.005.
Tatone C, Di Emidio G, Battaglia R, Di Pietro C. Building a human ovarian antioxidant CeRNA network ovanox: a bioinformatic perspective for research on redox-related ovarian functions and dysfunctions. Antioxid (Basel). 2024;13(9):1101. https://doi.org/10.3390/antiox13091101.
Huang X, Chen J, Li H, Cai Y, Liu L, Dong Q, et al. LncRNA SNHG12 suppresses adipocyte inflammation and insulin resistance by regulating the HDAC9/Nrf2 axis. FASEB J. 2024;38(13):e23794. https://doi.org/10.1096/fj.202400236RR.
Xia X, Zhang H, Xia P, Zhu Y, Liu J, Xu K, Yuan Y. Identification of Glycolysis-related LncRNAs and the novel LncRNA WAC-AS1 promotes Glycolysis and tumor progression in hepatocellular carcinoma. Front Oncol. 2021;11:733595. https://doi.org/10.3389/fonc.2021.733595.
Liu JB, Zhang JB, Yan XM, Xie PG, Fu Y, Fu XH, et al. DNA double-strand break-related competitive endogenous RNA network of noncoding RNA in bovine cumulus cells. Genes (Basel). 2023;14(2):290. https://doi.org/10.3390/genes14020290.
Elizur SE, Friedman Gohas M, Dratviman-Storobinsky O, Cohen Y. Pathophysiology mechanisms in fragile-X primary ovarian insufficiency. Methods Mol Biol. 2019;1942:165–71. https://doi.org/10.1007/978-1-4939-9080-1_14.
Alvarez-Mora MI, Agusti I, Wijngaard R, Martinez-Barrios E, Barcos T, Borras A, et al. Evaluation of FMR4, FMR5 and FMR6 expression levels as non-invasive biomarkers for the diagnosis of fragile X-associated primary ovarian insufficiency. J Clin Med. 2022;11(8):2186. https://doi.org/10.3390/jcm11082186.