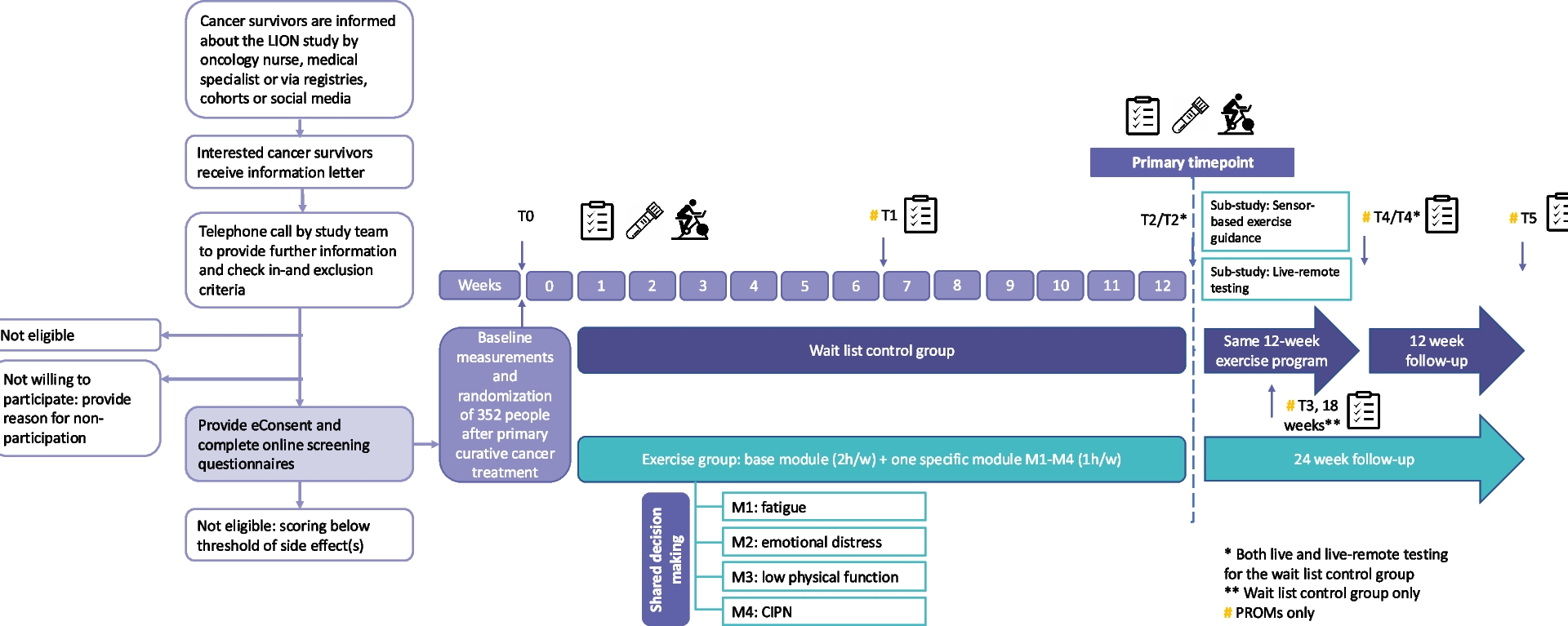
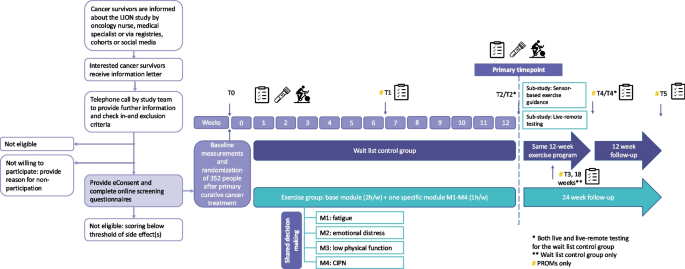
Study design
The LION study is a multinational randomized controlled trial (RCT) with two study groups: (1) an intervention group that receives a 12-week personalized, live-remote exercise program, and (2) a wait list control group that receives the same exercise program after the 12-week “control” period. Both groups will continue to receive usual care throughout the study period. The primary hypothesis is that participation in the 12-week live-remote exercise program will improve HRQoL and/or reduce participants’ most burdensome side effects at 12 weeks compared to the wait list control group.
This RCT is conducted at eight hospitals and study centers in five European countries and Australia: the Netherlands (University Medical Center Utrecht (UMCU, Sponsor) and the Netherlands Cancer Institute (NKI)), Germany (Heidelberg University Hospital/German Cancer Research Center (DKFZ)/National Center for Tumor Diseases (NCT) Heidelberg and German Sport University Cologne (DSHS)), Portugal (Associação de Investigação de Cuidados de Suporte em Oncologia (AICSO) and Unidade Local de Saude Gaia Espinho), Spain (Gipuzkoa Cancer Unit, OSID-Onkologikoa, BioGipuzkoa, Osakidetza (ONK)), Sweden (Karolinska Institutet (KI)), and Australia (Cabrini Health and Australian Catholic University (ACU)).
The study protocol was approved in December 2023 by the institutional review board of the University Medical Center Utrecht, the Netherlands, and subsequently by the local ethical review boards of all participating institutions. Written informed consent will be obtained from all participants. The study was registered with ClinicalTrials.gov on 13 Feb 2024 (NCT06270628). The first participant was included on 14 Feb 2024.
More information about organizational aspects of the trial can be found in Additional file 1.
Study sample
We plan to include 352 individuals who are approached within 12 to 52 weeks after completing primary cancer treatment, independent of their primary cancer diagnosis. To be eligible to participate in the study, individuals must meet the criteria outlined in Table 1. Medical in- and exclusion criteria are checked by an involved physician at the treating hospital.
Table 1 In- and exclusion criteria of the LION-RCTRecruitment and randomization
Participants are recruited in the abovementioned hospitals and study centers. Some centers have engaged additional recruitment sites. The study procedures are summarized in Fig. 1. Potentially eligible individuals are informed about the study by a member of the medical team (e.g., oncology nurse or medical specialist) during a regular follow-up visit or by email/letter/telephone/online call. In addition, national cancer registries/cohorts and social media (e.g., of national/local patient organizations) are used for recruitment. Interested individuals receive an information letter explaining the study aims and procedures. Subsequently, they are contacted by telephone to provide further information, answer questions and to check the inclusion and exclusion criteria. Potentially eligible individuals who choose not to participate in the LION-RCT are asked, but not required, to provide a reason for non-participation. Potentially eligible individuals who are willing to participate are asked to obtain exercise clearance for participation in the study from their treating physician or general practitioner. Once clearance is given, they are asked to provide online informed consent via Castor eConsent (Castor, The Netherlands). After providing consent, they complete online screening questionnaires to assess the presence and severity of the four side effects being targeted in this study.
Recruitment and LION-RCT procedures
To be eligible for the study, potential participants have to score above the threshold (Table 2) for at least one of the targeted side effects. Individuals who do not score above the threshold for any of the side effects are provided with country-specific resources related to exercising following a cancer diagnosis. Individuals who score above the threshold for multiple side effects meet with a member from the local research team to identify their most burdensome side effect through a shared decision-making process. Following this process, eligible participants are invited for their baseline visit (i.e., assessment of anthropometrics and physical fitness/performance, and blood draw).
Table 2 Pre-defined cut-off values for the side effects
After the baseline visit, participants are randomly allocated (1:1) to either the intervention or wait list control group by central computerized randomization using a blocked computer-generated sequence, effectively concealing the random allocation sequence. Castor EDC®, a cloud-based clinical data management platform, is used for randomization and data capture. Randomization is stratified by the participant’s most burdensome side effect, country and sex. Due to the nature of the intervention, blinding of participants and the study team to intervention assignment is not possible. However, data analysts will remain blinded to group allocation until the primary analyses have been completed. Participants randomized to the intervention group receive exercise instructions and an exercise kit at the end of their baseline visit. This kit includes the necessary exercise equipment to set-up their home exercise space: gym mat, dumbbell set with adjustable weights, resistance bands and an aerobic stepper. The wait list control group receives this kit 12 weeks post-baseline. All participants receive an activity tracker (Fitbit) at baseline.
Live-remote exercise program
The 12-week live-remote multimodal exercise program is delivered via Zoom. Each participant receives three exercise sessions per week. The sessions are broadcasted from one center in each participating country and supervised by a qualified exercise professional. To ensure standardization of the exercise program across broadcast centers, a train-the-trainer workshop was delivered. These workshops have been recorded and are part of the general study onboarding to ensure that staff recruited during the course of the study receives the same training.
Several sessions are offered at various fixed times per week, lasting 60–75 min each and involving a maximum of 10 participants. Exercise sessions open 15 min before the start of the session to facilitate social interaction. To tailor the intervention to the individual participants, a modularized approach is used (Fig. 2). Each participant attends three exercise sessions per week: two sessions focused on improving HRQoL (i.e., Base Module) and one session targeting the participant’s most burdensome side effect (i.e., Specific Module), as detailed further below. Participants are advised to be as active as possible on all remaining days of the week. At the start of the exercise program, participants receive the multi-language PREFERABLE app. This app is linked to the activity tracker and was initially developed to support unsupervised exercise in patients with cancer in the previous PREFERABLE-EFFECT study (NCT04120298) [21]. Originally, the app only included exercises that participants learned during the exercise program and can be performed at home. Some adaptations have been made to the app to suit the needs of the LION-RCT (i.e., adding educational resources and audio files for relaxation exercises in the Specific Modules). This app provides exercise support beyond the exercise program, during holidays and after the end of the intervention.
Overview of the base and specific modules of the LION exercise program
Prior to the first session of the live-remote exercise program, an exercise professional conducts a one-on-one live-remote intake session with each participant, during which the exercises, training equipment, and training documentation are explained. In addition, the exercise environment, technical connection, and relevant medical history of the participant are checked. As part of the safety plan, the participant is educated about exercising safely at home. The training location and emergency contact details are documented, and a flowchart outlining the process for requesting assistance in case of an emergency is also discussed. Participants are encouraged to invite family members, informal caregivers, and/or friends to the intake session, allowing them to ask questions and provide support as needed. To ensure continuity and safety during the live-remote exercise sessions, back-office assistance (e.g., administrative staff, research staff or clinical staff not involved in delivering the session) is available to all exercise professionals. This person can assist the exercise professional by joining the session or contacting a patient offline if, for example, an adverse event or technical issue occurs. Additionally, break-out rooms are available via Zoom, which allows the exercise professional or back-office assistant to discuss medical information or other sensitive issues with the participant in private.
Base module—HRQoL
The base module is based on the recent ACSM exercise guidelines for cancer survivors [7]. Each session starts with a 5–10 min warm up, consisting of mobilization and light intensity aerobic exercises. The warm-up is followed by 30 min of resistance training, during which seven to eight exercises that target the major muscle groups are performed using either one’s own body weight, dumbbells or resistance bands. Two sets of 8–12 repetitions per exercise are performed at moderate-to-vigorous intensity (rate of perceived exertion (RPE) 13–17). Each time the maximum number of repetitions is reached in both sets, and the participant’s condition allows, the intensity of the exercise is increased. Conversely, the intensity is reduced if the participant does not reach the minimum number of repetitions or in case of health problems. After the resistance training component, participants perform 20 min of continuous aerobic training, comprised of step aerobics at a moderate intensity (65–75% of age-predicted maximal heart rate; RPE 12–13). The intensity is monitored with the activity tracker and the RPE scale. Finally, a 5–10 min cool down consisting of stretching exercises is performed to conclude each session.
Specific module—fatigue
Participants who report fatigue as their most burdensome side effect receive this module as their third exercise session per week. Each session starts with breathing and joint mobilization exercises (10 min). The core of the session (40 min) consists of mind–body exercises with a focus on body-awareness. The mind–body exercises include postures and movements based on yoga and Tai Chi, as these exercise modalities have been proven to have a beneficial effect on cancer-related fatigue [22]. To make it easier for the participant to follow and fully immerse themselves in the movement, the same sequence of mind–body exercises is performed every session. Each session concludes with a relaxation exercise (body scan) for 10 min.
Specific module—emotional distress
Participants who report emotional distress as their most burdensome side effect receive this module as their third exercise session per week. Each session starts with breathing and joint mobilization exercises (10 min). The core of the session (35 min) consists of yoga-based exercises with a focus on body-awareness. Yoga-based exercises are included as prior evidence supports yoga as a beneficial exercise modality to reduce anxiety and depressive symptoms in cancer survivors [23]. To make it easier for the participant to follow and fully immerse themselves in the movement, the same sequence of exercises is performed in every session. Each session concludes with a 15-min audio-guided relaxation exercise, alternating exercises that include aspects from progressive muscle relaxation, body scan, and guided imagery.
Specific module—low physical functioning
Participants who report low physical functioning as their most burdensome side effect perform a third Base Module session, in line with the ACSM exercise guidelines [7].
Specific module—CIPN
Participants who report CIPN as their most burdensome side effect receive this module as their third exercise session per week. For these participants, the exercise kit also includes a foam pad, tennis ball, golf ball, massage ball, and therapeutic clay. The content of the module is based on emerging evidence and experiences from routine care that suggest beneficial effects of sensorimotor and balance exercises on CIPN [22, 24, 25]. Each session starts with a 5–10-min warm up, including mobilization, foot muscle activation, and body awareness exercises. The core of the session consists of balance and hand training (30 min) and agility training (15 min). The balance and hand training involves 4 rounds of static balance exercises and 4 rounds of hand exercises to improve fine-motor skills, proprioception and grip strength. The agility training involves 4 rounds of agility exercises. Each session is concluded with a 5–10-min cool down, including stretching exercises and massages with a ball. Participants in this module are also encouraged to perform some exercises of the Base Module with bare feet to provide extra sensorimotor stimulation.
Educational component
In addition to the live-remote exercise program, the intervention includes an educational component (Fig. 2). Educational resources are delivered via the app and a printout. The overall goal of the educational component is to improve exercise adherence throughout the intervention. In addition, side effect specific educational topics are included to improve the participants’ knowledge of their side effects, enable them to understand why exercise is important, promote behavior change, and support them in maintaining a physically active lifestyle post-intervention.
Wait list control group
Participants randomized to the wait list control group are asked to maintain their current physical activity behavior for the duration of the “control” period (12 weeks). They receive an activity tracker at baseline. After completion of the 12-week assessment, they are provided with the live-remote exercise program, following the same procedures as the intervention group. The most burdensome side effect as determined at baseline is checked with the participant again before the start of the exercise program, and a change is possible if symptoms have changed.
Sensor-based guidance (sub-study)
This sub-study primarily aims to evaluate the feasibility of integrating real-time wearable sensor data into live-remote exercise sessions. The objective is to complement the live-remote supervision with objective sensor data, enabling better informed exercise guidance and prescription. During the Base Module exercise session, participants wear electrophysiological sensors (Hexoskin) and an inertial measurement unit (Enode Pro) to assess movement parameters. During aerobic exercise, heart rate, step cadence, and breathing rate are monitored. During resistance exercise, repetition count and the mean concentric movement velocity of each repetition are tracked to guide resistance training based on the individual velocity loss. These data streams are available to the exercise professional in real-time, allowing them to adjust and cue exercise intensity accordingly. Additionally, nocturnal heart rate variability and sleep metrics are tracked following exercise sessions to retrospectively evaluate recovery. Participation in this sub-study is offered to wait list control group participants recruited at the German sites and is not mandatory. Participants who agree to take part in the sub-study exercise in dedicated training sessions that only involve participants wearing sensors. In case participants decline to participate in this sub-study, they proceed with the regular exercise program.
Study outcomes
Participants visit the study center for assessments at baseline, 12 weeks, and 24 weeks (wait list control group only), with 12 weeks being the primary time point. Patient-reported outcomes are assessed using online questionnaires, which participants either receive via Castor EDC shortly prior to the study center visit or complete during their visit, prior to the physical tests. Patient-reported outcomes are also assessed remotely at 6 weeks, 18 weeks (wait list control group only), 24 weeks (intervention group only), and 36 weeks. Additionally, participants receive phone calls at these time points to collect information on potential (serious) adverse events ((S)AEs), changes in medication, and health status (e.g., cancer recurrence). During the in-person visits, anthropometrics and physical fitness/performance are assessed and blood samples are drawn. To inform whether the intervention could be delivered entirely live-remote in routine clinical practice, we assess if live-remote testing is also feasible. Therefore, we perform live-remote physical fitness and function testing in all wait list control participants at 12 and 24 weeks as well.
Socio-demographic data are assessed at baseline with a study-specific questionnaire. Medical data are based on patient-report or retrieved from medical records. Personal data are coded and all data are handled in compliance with the General Data Protection Regulation (GDPR) (EU) 2016/679. Validity of the data is checked by an independent monitor.
Primary outcomes
The LION-RCT has two primary outcomes: HRQoL and the participant’s most burdensome side effect (i.e., cancer-related fatigue, emotional distress, low physical functioning, or CIPN).
HRQoL
HRQoL is assessed with the core questionnaire of the European Organization for Research and Treatment of Cancer (EORTC) that has been developed and validated for assessing HRQoL in patients with cancer (EORTC QLQ-C30). The summary score, which includes all original EORTC QLQ-C30 subscales excluding the global QoL score and financial difficulties score, is used as primary outcome [26, 27]. Scores range from 0 to 100 with a higher score indicating a better HRQoL.
Most burdensome side effect
The following questionnaires will be used to assess the side effects. Details on how the scores will be combined are provided in the statistical analyses section.
Cancer-related physical fatigue is assessed with the EORTC QLQ-FA12 questionnaire [28]. This is a 12-item questionnaire that assesses different dimensions of cancer-related fatigue. We use the physical fatigue dimension as primary outcome. Scores range from 0 to 100 with a higher score indicating higher levels of physical fatigue. Emotional distress is assessed with the Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS) [19], which combines the 9-item Patient Health Questionnaire depression scale (PHQ-9) and 7-item Generalized Anxiety Disorder scale (GAD-7). A composite score, ranging from 0 to 48, is used to assess the overall burden of anxiety and depression, with a higher score indicating a higher burden.
Physical functioning is measured using the 5-item EORTC QLQ-C30 physical functioning scale. To increase reliability for use in patients with higher levels of physical functioning, five items from the EORTC questionnaire item bank were added to the physical function scale (see Additional file 2 for the specific items). A domain-specific T-score will be calculated using the EORTC software and including all ten items. This T-score reflects the score of the participant relative to an age- and gender-matched European reference population, with 50 representing average physical functioning.
CIPN is assessed using the EORTC QLQ-CIPN20 questionnaire, which consists of 20 items addressing symptoms and the functional impact of CIPN [29]. A sum score of items 1–18, ranging from 0 to 100, is used to assess the overall CIPN burden, with a higher score indicating more severe symptoms [30].
The primary outcomes are assessed at all time points using the above-mentioned questionnaires. The same questionnaires are used to ascertain eligibility, except for CIPN (2 PRO-CTCAE items). Therefore, at baseline, they are only completed once, if the time window between eligibility screening and the baseline visit is less than 4 weeks. If the time window between eligibility screening and the baseline visit is more than 6 weeks, the participants are asked to repeat the questionnaires (Table 3).
Table 3 Overview of all assessments in the LION-RCTSecondary outcomesPatient-reported outcomes
Secondary outcome measures comprise the EORTC QLQ-C30 function and symptom scales, and the other fatigue dimensions of the EORTC QLQ-FA12 (emotional, cognitive, and total fatigue scores). We extended the social and role functioning scales of the EORTC QLQ-C30 by 2 items from the EORTC item bank (see Additional file 2 for the specific items). Sleep problems are assessed using the Pittsburgh Sleep Quality Index (PSQI), which contains 19 self-reported items assessing subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction over the past month [31]. In addition to the general pain score of the EORTC QLQ-C30, pain (i.e., joint and muscle pain) is evaluated using two specific items from the EORTC Survivorship questionnaire (EORTC QLQ-SURV100; see Additional file 2) [32]. These items are scored on a scale from 0 to 100, with higher scores indicating more pain, and will be analyzed separately. Fear of recurrence is assessed using five specific items from the EORTC QLQ-SURV100 (see Additional file 2). These items are summarized into two scores, health distress and symptom awareness, with a score ranging from 0 to 100 and a higher score indicating more fear of recurrence. Cognitive functioning is assessed with two subscales of the Functional Assessment of Cancer Therapy–cognitive functioning questionnaire (FACT-cog) [33]. The “Perceived Cognitive Impairment” subscale includes 20 items with a score ranging from 0 to 80. The “Impact on QoL” subscale includes 4 items with a score ranging from 0 to 16. A higher score indicates a better QoL. The 25-item Work Limitation Questionnaire (WLQ) assesses the extent to which health problems affect specific aspects of job performance and its impact on productivity [34]. An overall WLQ-index score can be calculated. This score ranges from 0 to 100 with a higher score indicating more impact of health problems on productivity. Body image is assessed using the 10-item Body Image Scale (BIS) [35]. A total score can be calculated, ranging from 0 to 30 with a higher score indicating more body image distress.
Physical activity
Self-reported physical activity levels are measured using the Modified Godin-Shephard Leisure Time Exercise Questionnaire, which consists of four items that assess the average frequency and duration of mild, moderate, and vigorous intensity aerobic activities, as well as moderate-to-vigorous intensity resistance exercises performed in bouts of at least 10 min during leisure time in a typical week [36, 37]. In addition, questions were added about exercise types and settings. The activity tracker is used to measure step count, heart rate, and physical activity minutes. Participants in both the intervention and wait list control group are instructed to wear the activity tracker throughout the study period, but at least during the 7 days following randomization and the 7 days preceding the 12-week, 24-week, and 36-week post-baseline assessments. Additionally, participants are asked to wear the activity tracker during live-remote exercise sessions and physical fitness testing.
Anthropometrics
Anthropometric data (i.e., body weight, height, waist-to-hip ratio) are measured in lightweight clothing without shoes.
Body composition
As add-on measurement, body composition (fat mass and fat free mass) is assessed in some centers using a whole body/segmental bio impedance analysis (BIA). Measurements are taken in a fasted state (no enteral intake for a minimum of 2 h), with an empty bladder, in a standing or lying position. BIA devices may differ between centers, but participants within each center are consistently measured on the same device.
Resting heart rate and blood pressure
Resting heart rate and blood pressure are measured in a seated position prior to the physical measurements.
Blood markers
Blood sampling is performed by vein puncture at baseline and 12 weeks post-baseline. Plasma and serum are derived from whole blood samples. Less than 30 ml of blood is collected per visit. In the 24 h prior to blood sampling, participants are instructed not to exercise vigorously or drink alcohol, and in the 2 h prior to blood sampling, they are asked to abstain from cigarettes, food, and drinks. Immediately after collection, a proportion of the blood sample is analyzed to assess full blood count indices such as leukocytes, hemoglobin, and C-reactive protein. The remaining blood is centrifuged and stored at < −70 °C at the local laboratories according to standardized procedures. Blood samples are transferred to the central biobank at the KI for analysis after the last sample has been collected locally.
Physical fitness tests (in-person)
The order of the in-person physical fitness tests is standardized. First, we assess balance and functional mobility and subsequently, functional and maximal muscle strength, aerobic capacity and maximum short exercise capacity (Table 3). All tests are performed according to standardized protocols. These protocols can be found in Additional file 3.
Physical fitness tests (live-remote)
The live-remote testing sessions are performed via Zoom. Prior to conducting the physical assessments, the researcher checks whether the participant’s testing environment is suitable and safe to conduct the tests. In addition, safety measures (i.e., recording participant’s address and emergency contact details) are discussed with the participant. The live-remote testing sessions are recorded to verify the correct execution of the tests after the sessions. For the different tests, the researcher instructs the participant to change the positioning of the camera to ensure an optimal view.
At the start of the live-remote testing sessions, the participant’s heart rate is measured using the activity tracker. The order of the tests is standardized. First, we assess balance and functional mobility and subsequently, muscle strength and aerobic capacity. All tests that overlap with the in-person setting (i.e., single leg stance, Timed Up and Go test, 30 s sit-to-stand, and Chester Step Test) are conducted according to the same standard operating procedures, as described in Additional file 3.
Additionally, a push-up test and a plank position holding time test is performed during the live-remote testing sessions to assess muscle strength [38, 39]. The positions of these exercise are demonstrated during the in-person visits. For the push-up test, participants are asked to complete as many push-ups on knees as possible, while maintaining proper form. The test is stopped when the participant is fatigued and stops the test or fails to maintain proper form. The number of correctly performed push-ups is recorded.
For the plank position holding time test, participants are asked to hold a static plank position as long as possible. The test is stopped when the participant is fatigued and stops the test or fails to maintain proper form. The time in plank position is recorded.
Cost-effectiveness
A cost-effectiveness analysis (CEA) will be conducted alongside the trial by comparing the costs and effects of the intervention. The EQ-5D-5L is used to assess effects on health across five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, each rated on five levels of severity [40]. This questionnaire is used to calculate quality-adjusted life years (QALYs). The analysis will be conducted from a societal perspective, including healthcare costs, patient and family costs, and productivity costs. Costs of the live-remote exercise program will be calculated bottom-up. Other direct costs are collected through questionnaires. A healthcare use questionnaire is used, which is based on the iMTA Medical Cost Questionnaire (iMCQ), including cost categories relevant to cancer survivors. Productivity losses are assessed using the modified Productivity Cost Questionnaire (iPCQ) [41].
Adherence
Adherence incorporates both attendance to the live-remote exercise sessions and compliance with the exercise prescription according to protocol. For each scheduled session, the exercise professional documents attendance in a case report form and records reasons for missed training sessions. An app (Castor Connect App) is used to capture compliance with all parts of the exercise program. Study participants record all exercise specifications (i.e., number of repetitions/rounds and, if applicable the weight or resistance band used, per exercise) and, if applicable, reasons for deviations from the protocol in the app. If participants prefer, they can also register compliance data on a paper form, in which case the data is entered into the app by the exercise trainer or study team.
Satisfaction
At 12 weeks (intervention group) and 24 weeks (wait list control group), we assess satisfaction with the live-remote exercise program, the exercise professionals, the activity tracker, educational resources and supporting exercise app by means of a study-specific questionnaire. In addition, participants who have completed the intervention are invited to provide more in-depth input on how the interaction between participants and the interaction between the exercise professional and participants could be optimized. This is done through a concept mapping approach. Participants can share their insights by either responding to two focus prompts, or by sorting and ranking the responses of others to these focus prompts. Responses are collected through the GroupWisdom application (Ithaca, NY, USA).
Sensor-based outcomes
The sensor-based sub-study will assess the feasibility, usability, and subjective experience with the technology and sensor-based exercise guidance, alongside adherence and technological reliability. In this context, usability and the experience of participants and exercise professionals regarding the technology use and sensor-based guidance are assessed using the System Usability Scale (SUS) and semi-structured interviews. Adherence is evaluated based on participant compliance with wearing the sensors (i.e., number of times sensors were worn during and after sessions). Additionally, adherence to the exercise prescription is analyzed, including the time spent within the prescribed heart rate zone (65–75%) during aerobic exercise, and the number of repetitions during resistance exercise. Technological feasibility is assessed through indicators such as device failures, connection errors, and problems documented by participants and exercise professionals.
Safety
All (serious) adverse events ((S)AE) are recorded according to the Exercise Harms Reporting Method (ExHaRM) [42]. First, (S)AEs are monitored and identified as follows: (1) Participants in both groups are asked by the study personnel about (S)AEs in a standardized manner during all follow-up visits; (2) Participants are asked by their exercise professional, before and after each live-remote exercise session, whether any (S)AEs occurred during or since the last exercise session; (3) Exercise professionals may observe a (S)AE during the exercise program; (4) (S)AE is spontaneously reported by participant between follow-up visits. Second, all (S)AEs, whether or not related to the study, are assessed and recorded by the exercise professional. The exercise professional indicates whether or not each event is related to the study. If unrelated, the adverse event is simply noted. If related, a detailed adverse event form is completed, including questions about the setting where the event occurred, its severity and actions taken (e.g., discontinuation or modification of intervention). Third, all (S)AEs are reviewed by an internal review board for relatedness to the exercise program or to the study measurements. All adverse events are coded using the Medical Dictionary for Regulatory Activities (MedDRA) to ensure standardized classification and grouping of similar events. SAEs are reported to the accredited ethical committee that approved the protocol, according to the requirements of that ethical committee.
Sample size
The sample size is based on the expected minimal improvement of either or both primary outcomes, i.e., HRQoL or the participant’s most burdensome side effect, from baseline to 12 weeks post-baseline relative to wait list control. To adjust for multiple testing, the Bonferroni-Holm method will be used. A Cochrane meta-analysis on RCTs with exercise interventions in breast cancer survivors after adjuvant chemo- and/or radiotherapy reported an effect size (95% CI) of 0.39 (0.21–0.57) for HRQoL and 0.34 (0.05–0.62) for depression, 0.33 (0.18–0.49) for perceived physical function, and 0.32 (0.18–0.47) for fatigue [43]. The subgroup of studies with combined aerobic and resistance exercises showed a higher effect size of 0.63 (0.08–1.19) for HRQoL and 0.47 (0.02–0.92) for fatigue. A meta-analysis comprising patients with mixed cancer entities after end of treatment also showed an effect size of 0.47 (0.29–0.65) for combined exercise on fatigue [44]. Further, higher effects are typically reached in studies enrolling only patients with the targeted symptom or side effect as well as with supervised compared to unsupervised exercise interventions [9, 11]. Hence, our planned intervention is expected to have effects that are in the higher range of previously reported studies. We performed a simulation study to calculate the power of the study considering that two primary outcomes will be tested and that a meta-analytic procedure will be used to obtain an average standardized effect size for participant’s most burdensome side effects. We assumed an effect size of 0.35 to obtain a conservative estimate for the required sample size. In the simulations, we assumed a correlation of 0.3 between the two primary outcomes. In case of a common effect size of 0.35 for HRQoL and participant’s most burdensome side effects in all four strata, a sample size of 280 individuals living beyond primary curative cancer treatment (n = 140 per study group) was found to yield 80% power to detect an effect on each individual primary outcome, with an overall alpha of 0.05. To account for a potential drop-out of around 20%, a total number of 352 individuals will be enrolled (n = 176 per study group). This sample size will also facilitate exploratory moderator and subgroup analyses to better understand which individuals benefit most from exercise.
Statistical analysis
Descriptive statistics will be used to characterize the study population at baseline. Questionnaire scores will be calculated according to published scoring manuals. All analyses will be performed according to the intention-to-treat principle, including all randomized participants in the groups to which they were assigned, irrespective of adherence.
A positive result at the 12-week assessment of any of the two primary outcomes (i.e., HRQoL and the participant’s most burdensome side effect) is of main interest. Statistical testing for the intervention effect will be done separately for each primary outcome. HRQoL will be assessed using the EORTC QLQ-C30 summary score. For the participant’s most burdensome side effect, a meta-analytic procedure will be used to obtain an average standardized effect size (ES) (see below for further details). To adjust for multiple testing, the Bonferroni-Holm method will be used to maintain an overall alpha level of 5%. Two-sided 95% confidence intervals for the effect estimates for both primary outcomes will be presented.
A mixed model for repeated measures will be used to assess the intervention effect on the primary outcome HRQoL, while taking the hierarchical structure of the data into account (participants nested within countries). Models will be adjusted for the baseline value of the outcome and stratification factors (i.e., sex, country and the participant’s most burdensome side effect) and will include participants for whom the outcome is observed at two or more timepoints. Time since completion of curative treatment will be added as a cofactor, if there is an imbalance between groups. The primary contrast will be the comparison of the mean outcomes at 12 weeks between the intervention and control group adjusted for the baseline value.
As the participant’s most burdensome side effect will differ between participants, inference for this primary endpoint in the whole study sample will be based on a pooled estimate of the stratum-specific standardized mean differences. A two-step procedure will be used. First, standardized ES will be calculated in the four strata separately (e.g., for all patients with fatigue as main side effect, an ES will be calculated). Subsequently, these four standardized ES’s will be pooled using a fixed-effect meta-analytic model. This pooled standardized ES can be interpreted as a stratum-size weighted average of the standardized ES for participant’s most burdensome side effect at 12 weeks.
The same analysis as for HRQoL will be performed for secondary outcomes assessed at more than 2 time points. Outcomes assessed at only two time points (i.e., physical fitness and performance) will be analyzed as between-group differences in outcomes using ANCOVA, adjusted for baseline values and stratification factors. All secondary analyses will be considered exploratory with statistical testing being done at the 5% significance level. For all secondary outcomes, ESs and 95% CIs are reported without p-values, according to the European Medicines Agency (EMA) guidelines. The statistical analysis plan is included in the IRB study protocol.
To assess whether a similar intervention effect is seen in the whole study sample or whether subgroups can be identified that benefit most from the intervention, potential moderators of the exercise effect will be investigated, e.g., for main side effect groups, sex, cancer type, or baseline fitness level. Further, to explore potential underlying mechanisms (e.g., inflammation) of exercise effects on the primary outcomes, mediation analyses will be performed.
Missing values of the primary outcome variables as well as all other patient-reported outcomes will be assumed to be Missing at Random (MAR) for primary analyses and dealt with using mixed models for repeated measures (HQRL) and multiple imputation (most burdensome side effect). Intercurrent events (e.g., death, cancer recurrence, non-adherence (i.e., < 80% of scheduled sessions due to external reasons), contamination) will be handled according to our study protocol.
Cost-utility analysis
In the cost-effectiveness evaluation, the net costs and effects incurred with both strategies (live-remote exercise program vs wait list control) will be compared at 12 weeks post-baseline (i.e., primary time point). The results of both cost and effect measurements will be integrated using cost-utility analyses. The analysis will be performed from a societal perspective with a time horizon of 12 weeks. Because of the short time horizon, discounting is considered redundant. In the cost-utility analysis, efficiency is expressed in terms of costs per QALY. Incremental costs and incremental effects, expressed in a ratio will be estimated. A probabilistic uncertainty analysis using bootstrapping will be performed. As it is unlikely that the full pay-back period of investments of live-remote training will be visible after a follow-up of 12 weeks, we will extrapolate findings of the economic evaluation alongside in a model-based study and determine the potential cost-effectiveness at 36 weeks and a lifetime time horizon. For this model-based study, we will combine the trial data with literature data and expert opinion scenarios.
Analysis of live-remote testing
Agreement between live-remote and in-person tests will be determined with a Bland and Altman analysis and by using intraclass correlations (ICC) two-way mixed-effects models with absolute agreement since each measurement from the participant will be rated by the same assessor. Interpretations of the ICCs will be based on Koo and Li’s guidelines [45]: ICC values < 0.5 = poor reliability; 0.5–0.75 = moderate reliability; 0.75–0.9 = good reliability; and > 0.9 = excellent reliability.
Analysis of the sensor-based sub-study
Feasibility outcomes related to usability and experience scores will be presented as mean ± SD along with 95% confidence intervals. Qualitative data collected from semi-structured interviews with participants and exercise professionals will be analyzed using thematic analysis to explore their subjective experiences, perceptions of usability, and detailed feedback regarding the feasibility of the technology and sensor-based exercise guidance.
Concept mapping analysis
Statements generated and ranked by participants will be further analyzed using cluster analysis. This results in a visual map of related aspects, aggregated at a group level. This so-called “point-cluster” map is then used to identify and name overarching concepts. For each overarching concept, researchers will translate statements into actionable features and rank these according to importance and feasibility. This results in a “Go-zone map” per focus area of interest (i.e., group interaction and patient-trainer interaction), identifying which features are both feasible and important to pursue for optimizing delivery of the live-remote exercise intervention in future implementation.