Gynecologic malignancies—particularly ovarian, endometrial, and cervical cancers—remain major contributors to cancer morbidity and mortality, driven by late diagnosis, high relapse rates, and therapy resistance. Over the past decade, two drug classes have meaningfully shifted outcomes in selected populations: immune checkpoint inhibitors (PD-1/PD-L1 blockade) and PARP inhibitors. Each class, however, delivers its most consistent benefit in biomarker-defined subsets (e.g., dMMR/MSI-H for immunotherapy; BRCA/HRD for PARP inhibition).
A growing body of preclinical and translational work suggests these therapies may be mechanistically complementary: PARP inhibition increases DNA damage and can activate innate immune sensing (cGAS–STING), promote interferon signaling, and enhance tumor immunogenicity—an effect that may be amplified by PD-1/PD-L1 blockade.
Methods and Study Design
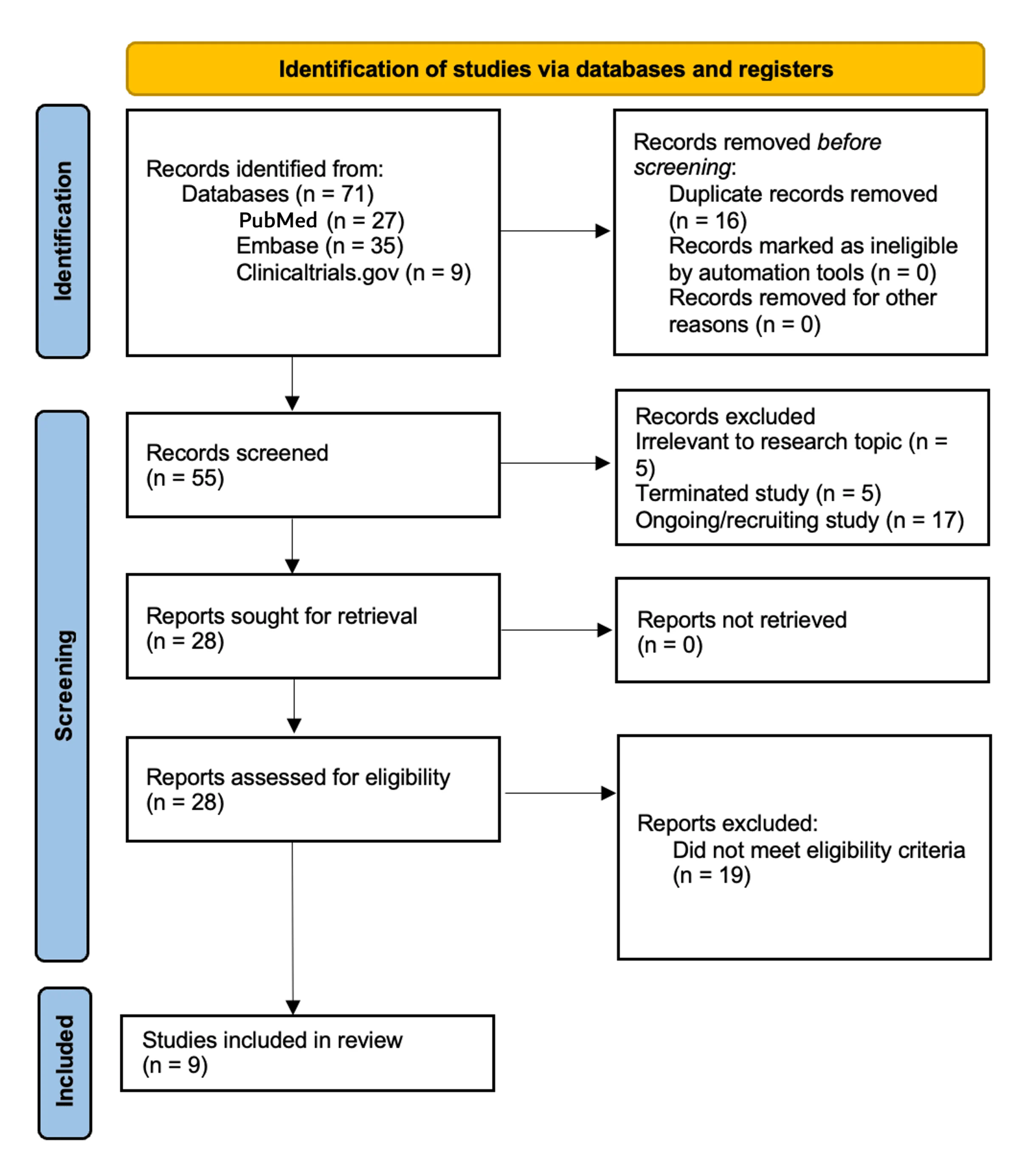
This publication is a structured narrative review. The authors searched MEDLINE, Embase, and ClinicalTrials.gov(Jan 1, 2015–Aug 24, 2025) for interventional trials testing a PARP inhibitor + anti–PD-1/PD-L1 agent in ovarian/fallopian tube/primary peritoneal, endometrial, or cervical cancer.
Key eligibility features:
Included studies required ≥1 efficacy endpoint (ORR, PFS, and/or OS) plus safety in a gynecology-only cohort (≥20 evaluable patients, or any phase III).
Triplets were allowed if the third agent was non-cytotoxic (e.g., bevacizumab).
Regimens with concurrent cytotoxic chemotherapy in the investigational combination were excluded to reduce confounding from overlapping myelosuppression.
Nine studies met criteria: 1 phase III and 8 phase I/II trials.
Results
Ovarian cancer: the most compelling setting (especially BRCA/HRD)
Across recurrent ovarian cancer studies, signals of benefit were most consistent in BRCA/HRD biology:
Niraparib + pembrolizumab demonstrated modest overall activity, with more durable responses enriched in HRD-positive tumors.
Olaparib + durvalumab showed strong activity in gBRCA platinum-sensitive relapse, consistent with a PARP-sensitive disease context.
Adding bevacizumab appeared to broaden benefit in non-BRCA cohorts in selected studies, supporting an immunomodulatory/vascular-normalization rationale for non-cytotoxic triplets.
Frontline maintenance: randomized evidence is not supportive
A key finding from the review is that in newly diagnosed ovarian cancer, PARP+IO has not proven superior:
Rucaparib + nivolumab maintenance failed to improve PFS versus rucaparib alone, highlighting the gap between early-phase signals and phase III performance in an unselected frontline setting.
Endometrial cancer: limited activity overall, signals confined to selected subgroups
In largely pMMR or biomarker-unselected endometrial cancer:
Olaparib + durvalumab and talazoparib + avelumab showed modest activity, with signals primarily appearing in biomarker-enriched subsets (e.g., HRR alterations).
This fits the broader pattern that endometrial cancer immunotherapy benefit is strongest in dMMR/MSI-H, where checkpoint blockade alone is often effective.
Safety and Tolerability
Toxicities largely reflected expected class effects:
PARP-driven myelosuppression (anemia, thrombocytopenia, neutropenia)
Immune-related adverse events consistent with checkpoint inhibition
Overall, most toxicities were described as manageable with standard algorithms, but combination approaches can increase treatment burden and monitoring requirements, particularly in triplets.
Insights
The synergy hypothesis is biologically strong, but clinical benefit is highly context-dependent.
The most credible efficacy signals cluster in ovarian cancer, especially BRCA/HRD and platinum-sensitivesettings, where the PARP “backbone” is inherently active.
Frontline maintenance intensification remains unproven, and randomized data to date do not support routine PARP+IO maintenance in unselected populations.
In endometrial cancer, the combination does not appear broadly transformative outside molecularly selectedgroups.
Key Takeaway Messages
Best-fit indication: ovarian cancer, particularly BRCA/HRD tumors; selected non-BRCA cases may benefit from non-cytotoxic triplets (e.g., bevacizumab-containing strategies).
Not practice-confirmed: frontline ovarian maintenance benefit remains unproven based on phase III evidence summarized here.
Endometrial cancer: activity is modest and likely requires biomarker-guided selection.
Future progress depends on biomarker-driven enrollment, rational partners, and optimized sequencing rather than broad, unselected combinations.
Conclusion
PD-1/PD-L1 blockade combined with PARP inhibition remains a promising but selective strategy in gynecologic oncology. Current evidence supports the concept most strongly in ovarian cancer, especially where BRCA/HRD biologyand platinum sensitivity suggest a robust PARP foundation. By contrast, frontline maintenance benefit is not established, and endometrial activity appears limited outside selected molecular contexts. The field now needs biomarker-enriched randomized trials, clearer sequencing strategies, and tolerability-aware regimens to define where this approach becomes truly practice-changing.
You Can Read All Article here

