Nano Suspension Market Forecast and Outlook 2025 to 2035
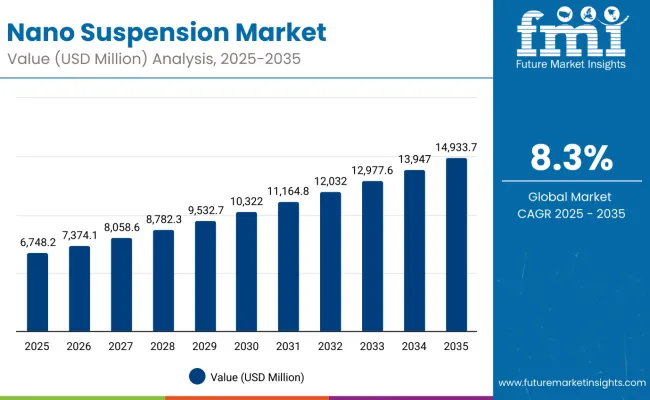
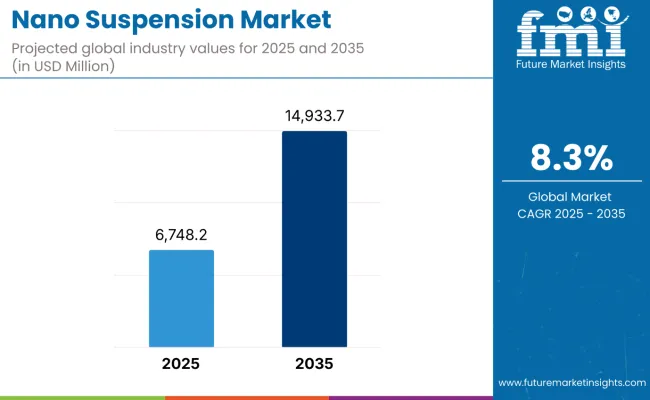
The global nano suspension market is projected to grow from USD 6,748.2 million in 2025 to approximately USD 14,933.7 million by 2035, recording an absolute increase of USD 8,185.5 million over the forecast period. This translates into a total growth of 121.3%, with the market forecast to expand at a compound annual growth rate (CAGR) of 8.3% between 2025 and 2035.
Quick Stats for Nano Suspension Market
Nano Suspension Market Value (2025): USD 6,748.2 million
Nano Suspension Market Forecast Value (2035): USD 14,933.7 million
Nano Suspension Market Forecast CAGR: 8.3%
Leading Product Type in Nano Suspension Market: Injectable Nano Suspensions (45.5%)
Key Growth Regions in Nano Suspension Market: North America, Europe, and Asia Pacific
Key Players in Nano Suspension Market: Bristol Myers Squibb, Bayer AG, Novartis AG, AbbVie Inc., NanoCarrier Co., Ltd., Phosphorex (MilliporeSigma), Evonik Industries AG, Capsulution AG, Abbott Laboratories
Nano Suspension Market Key Takeaways
Metric
Value
Estimated Value in (2025E)
USD 6,748.2 million
Forecast Value in (2035F)
USD 14,933.7 million
Forecast CAGR (2025 to 2035)
8.3%
The overall market size is expected to grow by nearly 2.2X during the same period, supported by increasing demand for enhanced bioavailability solutions, rising adoption of nanotechnology in pharmaceuticals, and growing focus on targeted drug delivery systems.
Between 2025 and 2030, the nano suspension market is projected to expand from USD 6,748.2 million to USD 9,871.4 million, resulting in a value increase of USD 3,123.2 million, which represents 38.2% of the total forecast growth for the decade. This phase of growth will be shaped by rising adoption of nanotechnology in drug formulation, increasing demand for personalized medicine approaches, and growing penetration of nano suspension technologies in emerging therapeutic areas. Pharmaceutical companies are expanding their nano suspension portfolios to address the growing demand for enhanced drug delivery solutions for complex therapeutic conditions.
From 2030 to 2035, the market is forecast to grow from USD 9,871.4 million to USD 14,933.7 million, adding another USD 5,062.3 million, which constitutes 61.8% of the overall ten-year expansion. This period is expected to be characterized by expansion of specialized pharmaceutical manufacturing facilities, integration of advanced nanotechnology platforms with drug development processes, and development of personalized nano suspension formulations. The growing adoption of precision medicine and regulatory approvals will drive demand for clinically proven nano suspension technologies with enhanced efficacy and safety profiles.
Between 2020 and 2025, the nano suspension market experienced robust expansion, driven by increasing recognition of bioavailability challenges in pharmaceutical development and growing acceptance of nanotechnology-based solutions. The market developed as pharmaceutical companies recognized the need for advanced formulation technologies to address poorly water-soluble drugs and complex delivery requirements. Clinical research and regulatory approvals began emphasizing the importance of nano suspension technologies in achieving better patient outcomes for challenging therapeutic applications.
Why is the Nano Suspension Market Growing?
Market expansion is being supported by the increasing prevalence of poorly water-soluble drugs in pharmaceutical pipelines and the corresponding demand for more effective formulation approaches. Modern pharmaceutical companies are increasingly focused on nano suspension technologies that can enhance bioavailability, improve therapeutic efficacy, and reduce dosing frequency.
The proven effectiveness of nano suspension formulations in addressing solubility challenges and enabling targeted drug delivery makes them essential components of comprehensive pharmaceutical development strategies.
The growing emphasis on personalized medicine and precision drug delivery is driving demand for tailored nano suspension formulations that address individual patient needs and specific therapeutic targets. Healthcare provider preference for advanced formulation technologies that can improve patient compliance and therapeutic outcomes is creating opportunities for innovative product development.
The rising influence of regulatory guidelines supporting nanotechnology applications and evidence-based formulation approaches is also contributing to increased adoption of nano suspension technologies across different therapeutic areas and patient populations.
Segmental Analysis
The market is segmented by product type, application type, end user, and region. By product type, the market is divided into injectable nano suspensions, oral nano suspensions, topical nano suspensions, inhalation nano suspensions, and others. Based on application type, the market is categorized into oncology, infectious diseases (incl. TB, fungal), neurology, cardiovascular & metabolic disorders, dermatology, and others (ophthalmology, rare diseases).
In terms of end user, the market is segmented into pharmaceutical & biopharma companies, specialty clinics & hospitals, research institutes & universities, and contract manufacturing organizations (CMOs/CDMOs). Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
By Product Type, Injectable Nano Suspensions Segment Accounts for 45.5% Market Share
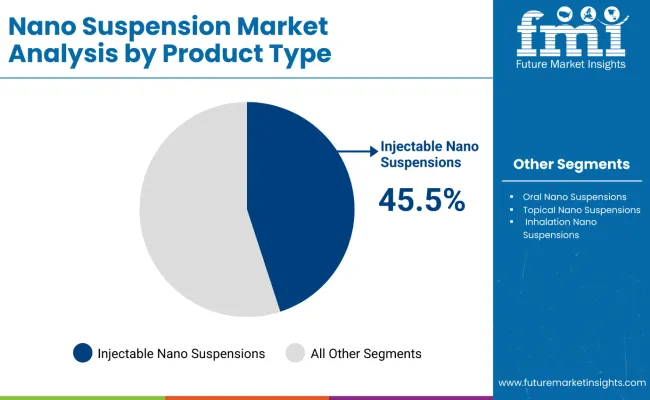
The injectable nano suspensions segment is projected to account for 45.5% of the nano suspension market in 2025, reaffirming its position as the category’s dominant product type. Pharmaceutical companies increasingly recognize the superior bioavailability and targeted delivery benefits of injectable nano suspension formulations, particularly for oncology, infectious diseases, and complex therapeutic applications. This product type addresses critical formulation challenges while providing enhanced therapeutic coverage and improved patient outcomes.
This product segment forms the foundation of most advanced drug delivery protocols, as it represents the most clinically validated and widely accepted nanotechnology approach in pharmaceutical development. Regulatory approvals and extensive clinical research continue to strengthen confidence in these formulations. With increasing recognition of the complexity of drug delivery requirements for poorly soluble compounds, injectable nano suspensions align with both acute treatment and long-term therapeutic management goals. Their broad therapeutic utility across multiple applications ensures sustained market dominance, making them the central growth driver of nano suspension technology demand.
By Application Type, Oncology Segment Accounts for 30.6% Market Share
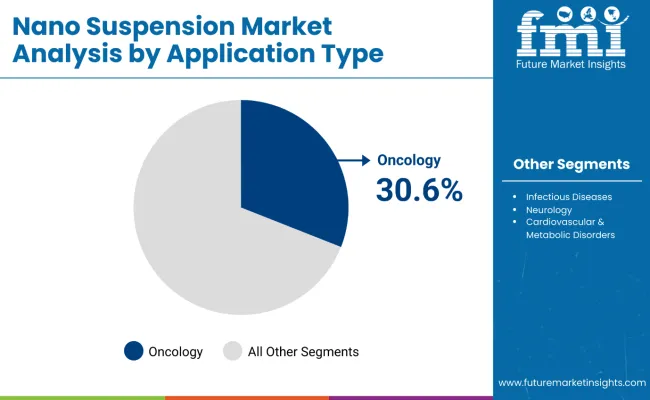
Oncology is projected to represent 30.6% of nano suspension demand in 2025, underscoring its role as the primary application driving nanotechnology adoption in pharmaceutical development. Healthcare providers and pharmaceutical companies recognize that cancer treatment’s complex therapeutic requirements, including targeted delivery, enhanced bioavailability, and reduced systemic toxicity, often require advanced formulation approaches that conventional delivery systems cannot adequately address. Nano suspension technologies offer enhanced efficacy in managing drug solubility challenges and improving therapeutic index.
The segment is supported by the critical nature of cancer treatment requiring precise drug delivery and the growing recognition that nano suspension approaches can improve therapeutic outcomes and reduce adverse effects. Additionally, pharmaceutical companies are increasingly adopting evidence-based development strategies that leverage nanotechnology for optimal oncology drug performance. As clinical understanding of cancer treatment complexity advances, nano suspension technologies will continue to play a crucial role in comprehensive therapeutic strategies, reinforcing their essential position within the pharmaceutical market.
By End User, Pharmaceutical & Biopharma Companies Segment Accounts for 52.1% Market Share
The pharmaceutical & biopharma companies segment is forecasted to contribute 52.1% of the nano suspension market in 2025, reflecting the primary role of pharmaceutical manufacturers in nanotechnology adoption and advanced drug development.
Companies developing complex therapeutic compounds rely on sophisticated nano suspension technologies for formulation optimization and enhanced drug delivery, making pharmaceutical & biopharma companies the cornerstone of nano suspension technology utilization. This segment provides essential capabilities including formulation development, clinical testing, and regulatory compliance support.
The segment benefits from established research and development infrastructure, manufacturing capabilities, and regulatory expertise that enable comprehensive nano suspension technology implementation. Pharmaceutical & biopharma companies also offer advantages in terms of clinical trial management, regulatory pathway navigation, and commercial manufacturing scale-up.
With growing emphasis on advanced drug delivery systems and precision medicine applications, pharmaceutical & biopharma companies serve as critical drivers of nano suspension technology innovation and adoption, making them fundamental catalysts of market growth and technological advancement.
What are the Drivers, Restraints, and Key Trends of the Nano Suspension Market?
The nano suspension market is advancing rapidly due to increasing recognition of bioavailability challenges in pharmaceutical development and growing demand for advanced drug delivery approaches. However, the market faces challenges including complex manufacturing processes, potential for increased production costs, and concerns about regulatory compliance for nanotechnology applications. Innovation in formulation technologies and personalized delivery protocols continue to influence product development and market expansion patterns.
Expansion of Contract Manufacturing and Specialized Production Facilities
The growing adoption of specialized pharmaceutical manufacturing facilities is enabling more sophisticated nano suspension production and quality control. Contract manufacturing organizations offer comprehensive formulation services, including particle size optimization and stability testing, that are particularly important for complex nano suspension formulations. Specialized production channels provide access to advanced manufacturing technologies that can optimize nano suspension development and commercial production.
Integration of Advanced Analytics and Process Control Systems
Modern pharmaceutical companies are incorporating advanced analytical technologies such as real-time particle monitoring, automated quality control systems, and process analytical technology to enhance nano suspension manufacturing. These technologies improve product consistency, enable real-time process optimization, and provide better quality assurance throughout production. Advanced analytical platforms also enable personalized formulation development and early identification of potential formulation challenges or stability issues.
Analysis of Nano Suspension Market by Key Country
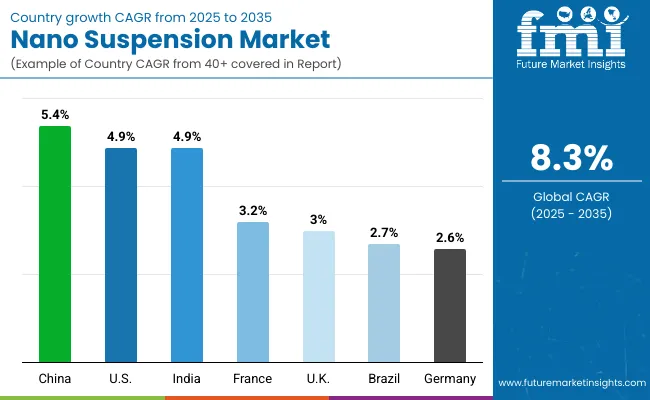
Country
CAGR (2025 to 2035)
India
4.9%
China
5.4%
Brazil
2.7%
USA
4.9%
France
3.2%
UK
3.0%
Germany
2.6%
The nano suspension market is experiencing varied growth globally, with India and the USA both leading at a 4.9% CAGR through 2035, driven by expanding pharmaceutical manufacturing capabilities, increasing nanotechnology adoption, and growing investment in advanced drug delivery systems. China follows at 5.4%, supported by pharmaceutical industry expansion, increasing research and development activities, and expanding manufacturing infrastructure.
Brazil shows growth at 2.7%, emphasizing improved pharmaceutical manufacturing capabilities and nanotechnology adoption. Europe records 4.2% growth, focusing on advanced manufacturing technologies and comprehensive pharmaceutical development systems. France shows 3.2% growth, representing established pharmaceutical infrastructure with continued innovation in nanotechnology applications.
The report covers an in-depth analysis of 40+ countries; seven top-performing countries are highlighted below.
China Demonstrates Strong Market Potential with Manufacturing Infrastructure Development
Revenue from nano suspensions in China is projected to exhibit robust growth with a CAGR of 5.4% through 2035, driven by ongoing pharmaceutical industry expansion and increasing adoption of advanced manufacturing technologies. The country’s expanding pharmaceutical manufacturing infrastructure and growing investment in nanotechnology research are creating significant opportunities for nano suspension technology adoption. Major international and domestic pharmaceutical companies are establishing comprehensive manufacturing networks to serve the growing demand for advanced drug delivery solutions across urban and developing regions.
Government initiatives supporting pharmaceutical innovation and nanotechnology development are driving demand for advanced formulation technologies throughout major industrial and research centers.
Pharmaceutical industry development and academic collaboration are supporting appropriate utilization of nano suspension technologies among pharmaceutical manufacturers and research institutions nationwide.
India Demonstrates Exceptional Market Potential with Pharmaceutical Industry Growth
Revenue from nano suspensions in India is expanding at a CAGR of 4.9%, supported by increasing pharmaceutical manufacturing capabilities, growing nanotechnology adoption, and expanding biotechnology sector presence. The country’s large pharmaceutical industry and increasing investment in advanced manufacturing technologies are driving demand for innovative nano suspension solutions. International pharmaceutical companies and domestic manufacturers are establishing development capabilities to serve the growing demand for advanced drug delivery technologies.
Rising investment in pharmaceutical research and development and expanding manufacturing infrastructure are creating opportunities for advanced nanotechnology applications across pharmaceutical and biotechnology sectors.
Growing pharmaceutical industry development and technology transfer initiatives are supporting increased adoption of nano suspension technologies among manufacturers requiring advanced formulation solutions.
United States Maintains Market Leadership with Advanced Technology Infrastructure
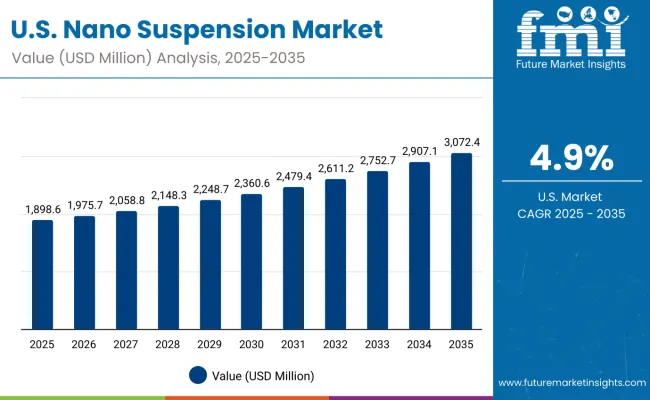
Demand for nano suspensions in the USA is projected to grow at a CAGR of 4.9%, supported by well-established pharmaceutical research infrastructure and advanced manufacturing capabilities. American pharmaceutical companies consistently utilize nano suspension technologies for complex drug development and advanced therapeutic applications. The market is characterized by mature technology platforms, comprehensive regulatory frameworks, and established relationships between pharmaceutical companies and nanotechnology providers.
Advanced research and development capabilities and regulatory expertise are supporting continued utilization of proven nano suspension technologies throughout pharmaceutical industry sectors.
Specialized manufacturing networks and biotechnology companies are facilitating access to nano suspension technologies while ensuring appropriate quality standards and regulatory compliance.
Brazil Shows Promising Growth with Pharmaceutical Sector Development
Revenue from nano suspensions in Brazil is projected to grow at a CAGR of 2.7% through 2035, driven by pharmaceutical sector development, increasing adoption of advanced manufacturing technologies, and growing recognition of nanotechnology importance. Brazilian pharmaceutical companies are increasingly adopting nano suspension approaches for complex formulation challenges, supported by expanding manufacturing capabilities and improved technology accessibility.
Pharmaceutical industry development and technology transfer programs are supporting increased availability of nano suspension technologies across diverse manufacturing applications.
Growing collaboration between international pharmaceutical companies and local manufacturers is enhancing technological capabilities and clinical expertise in nano suspension development.
United Kingdom Demonstrates Stable Growth with Pharmaceutical Excellence Framework
Revenue from nano suspensions in the UK is projected to grow at a CAGR of 3.0% through 2035, supported by pharmaceutical excellence frameworks and comprehensive technology development guidelines that facilitate appropriate use of nano suspension technologies for complex formulation applications. British pharmaceutical companies consistently utilize established protocols for nano suspension development, emphasizing product quality and manufacturing optimization within integrated development systems.
Pharmaceutical development pathways and regulatory guidelines are supporting systematic adoption of proven nano suspension technologies across pharmaceutical manufacturing and biotechnology sectors.
Industry education programs and technology research initiatives are maintaining high standards for nano suspension utilization and product quality assurance.
Germany Anchors Growth with Established Pharmaceutical Manufacturing Infrastructure
Revenue from nano suspensions in Germany is projected to grow at a CAGR of 2.6% through 2035, supported by the country’s well-established pharmaceutical manufacturing infrastructure, comprehensive quality systems, and systematic approach to advanced technology adoption. German pharmaceutical companies emphasize evidence-based nano suspension utilization within structured manufacturing frameworks that prioritize product quality and regulatory compliance.
Comprehensive manufacturing system coordination and regulatory oversight are supporting stable utilization of nano suspension technologies across diverse pharmaceutical applications and manufacturing facilities.
Academic research centers and industrial institutions are maintaining leadership in nano suspension technology research and manufacturing protocol development for optimal product outcomes.
France Maintains Steady Market Development with Integrated Pharmaceutical Approach
Revenue from nano suspensions in France is projected to grow at a CAGR of 3.2% through 2035, supported by the country’s comprehensive pharmaceutical system, established manufacturing infrastructure, and systematic approach to advanced technology development. French pharmaceutical companies emphasize evidence-based nano suspension utilization within integrated development frameworks that prioritize product innovation and manufacturing excellence.
Centralized pharmaceutical system coordination and comprehensive technology support are supporting stable access to nano suspension technologies across diverse manufacturing applications and research settings.
Academic research centers and industrial institutions are maintaining leadership in nano suspension research and manufacturing practice development.
Europe Market Split by Country
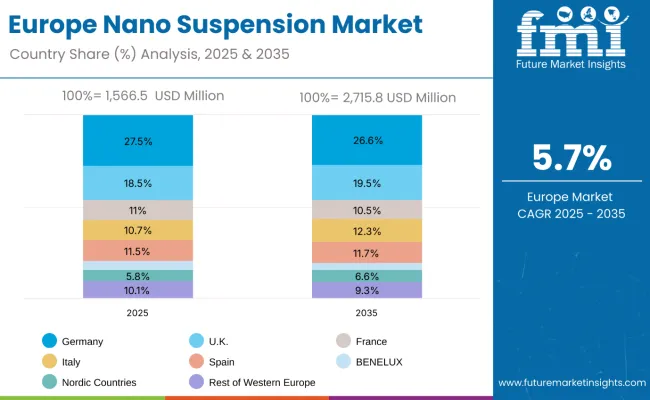
The nano suspension market in Europe is projected to expand steadily through 2035, supported by increasing adoption of advanced formulation technologies, rising demand for enhanced drug delivery systems, and ongoing innovation in nanotechnology applications.
Germany will continue to lead the regional market, accounting for 27.5% in 2025 and decreasing slightly to 26.6% by 2035, supported by strong pharmaceutical manufacturing base, regulatory expertise, and robust technology infrastructure. The United Kingdom follows with 18.5% in 2025, increasing to 19.5% by 2035, driven by pharmaceutical excellence initiatives, advanced research capabilities, and expanding biotechnology networks.
France holds 11.0% in 2025, decreasing to 10.5% by 2035 as pharmaceutical companies expand manufacturing capabilities and demand grows for specialized formulation technologies. Italy contributes 10.7% in 2025, rising to 12.3% by 2035, supported by strong pharmaceutical manufacturing programs and growing technology adoption. Spain represents 11.5% in 2025, maintaining stability at 11.7% by 2035, underpinned by strengthening pharmaceutical manufacturing capabilities and technology development programs.
BENELUX markets together account for 5.0% in 2025, decreasing to 3.6% by 2035, supported by innovation-friendly regulatory frameworks and academic-industry collaboration. The Nordic countries represent 5.8% in 2025, increasing to 6.6% by 2035, with demand fueled by progressive pharmaceutical systems and early adoption of advanced manufacturing technologies. The Rest of Western Europe represents 10.1% in 2025, decreasing to 9.3% by 2035, as larger core markets capture a greater share of investment, technology development, and adoption of nano suspension protocols.
Japan Segmental Share by Country
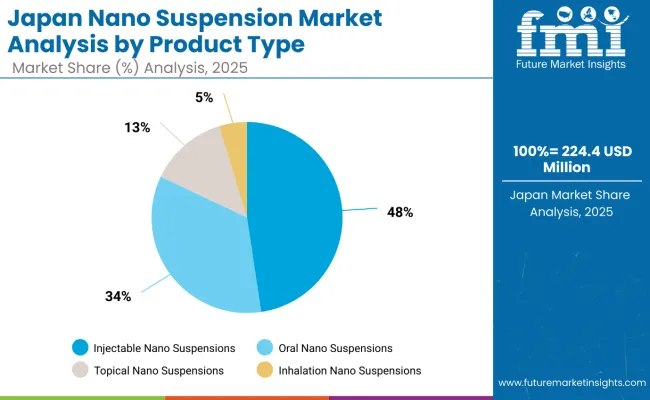
The nano suspension market in Japan is set to remain diversified across several product types in 2025, reflecting manufacturing preferences and evolving technology adoption patterns. Injectable Nano Suspensions dominate with a 47.7% share in 2025, supported by their central role in advanced drug delivery applications, oncology treatments, and complex therapeutic formulations.
Oral Nano Suspensions represent 34.4% in 2025, gaining traction from pipeline developments targeting enhanced bioavailability and patient compliance benefits. Topical Nano Suspensions hold 13.3%, sustained by their widespread use for dermatological applications and localized drug delivery requirements.
Meanwhile, Inhalation Nano Suspensions account for 4.7%, driven by specialized applications in respiratory medicine and targeted pulmonary delivery systems. The market composition reflects the growing role of advanced formulation technologies and precision delivery approaches entering clinical practice.
South Korea Segmental Share by Country
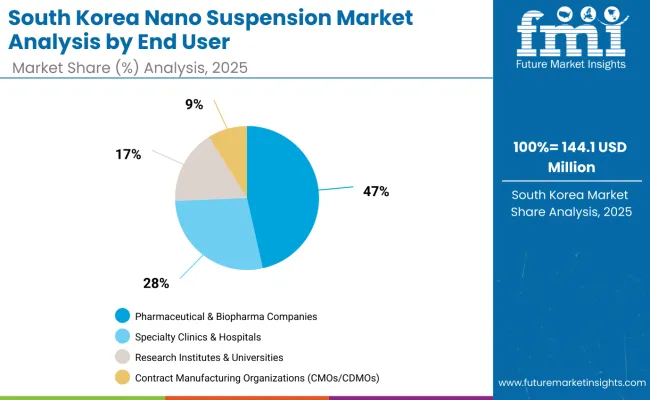
The nano suspension market in South Korea in 2025 is shaped by strong demand across core end user categories, reflecting both industrial capabilities and technology adoption patterns. Pharmaceutical & Biopharma Companies account for the largest share at 46.5%, supported by robust pharmaceutical manufacturing sector, advanced technology adoption, and increasing investment in nanotechnology applications.
Specialty Clinics & Hospitals follow with 27.9%, driven by growing adoption of advanced therapeutics, heightened focus on precision medicine, and wider utilization of nanotechnology-enhanced drug delivery systems. Research Institutes & Universities contribute 16.7%, supported by established research capabilities and integration of academic research with industrial applications.
Contract Manufacturing Organizations (CMOs/CDMOs) hold 8.9%, reflecting growing demand for specialized manufacturing services and advanced formulation capabilities. This distribution highlights opportunities for continued technology development and expanded industrial adoption across pharmaceutical and biotechnology sectors.
Competitive Landscape of Nano Suspension Market
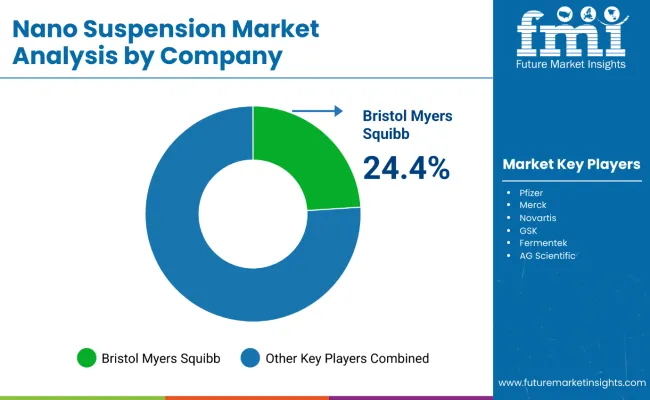
The nano suspension market is characterized by competition among established pharmaceutical companies, specialized nanotechnology firms, and contract manufacturing organizations. Companies are investing in research and development, manufacturing capabilities, strategic partnerships, and technology advancement to deliver effective, scalable, and accessible nano suspension solutions. Technology development, clinical validation, and manufacturing optimization strategies are central to strengthening product portfolios and market presence.
Bristol Myers Squibb leads the market with 24.4% global value share, offering clinically-proven nano suspension technologies with a focus on oncology and complex therapeutic applications. Bayer AG provides comprehensive pharmaceutical solutions with emphasis on advanced formulation technologies and manufacturing excellence. Novartis AG focuses on innovative drug delivery systems and specialized nanotechnology platforms. AbbVie Inc. delivers established pharmaceutical products with strong clinical evidence and advanced formulation capabilities.
NanoCarrier Co., Ltd. operates with focus on specialized nanotechnology solutions and innovative delivery systems for pharmaceutical applications. Phosphorex (MilliporeSigma) provides comprehensive nanotechnology platforms and manufacturing solutions across multiple therapeutic areas. Evonik Industries AG specializes in advanced materials and formulation technologies for nano suspension applications. Capsulution AG focuses on specialized encapsulation technologies and customized nano suspension solutions. Abbott Laboratories provides established pharmaceutical formulations and technology platforms to enhance market accessibility and patient access to advanced nano suspension treatments.
Key Players in the Nano Suspension Market
Bristol Myers Squibb
Bayer AG
Novartis AG
AbbVie Inc.
NanoCarrier Co., Ltd.
Phosphorex (MilliporeSigma)
Evonik Industries AG
Capsulution AG
Abbott Laboratories (legacy formulations)
Scope of the Report
Items
Values
Quantitative Units (2025)
USD 6,748.2 Million
Product Type
Injectable Nano Suspensions, Oral Nano Suspensions, Topical Nano Suspensions, Inhalation Nano Suspensions, Others
Application Type
Oncology, Infectious Diseases (incl. TB, fungal), Neurology, Cardiovascular & Metabolic Disorders, Dermatology, Others (ophthalmology, rare diseases)
End User
Pharmaceutical & Biopharma Companies, Specialty Clinics & Hospitals, Research Institutes & Universities, Contract Manufacturing Organizations (CMOs/CDMOs)
Regions Covered
North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa
Countries Covered
United States, Canada, United Kingdom, Germany, France, China, Japan, South Korea, India, Brazil, Australia and 40+ countries
Key Companies Profiled
Bristol Myers Squibb, Bayer AG, Novartis AG, AbbVie Inc., NanoCarrier Co., Ltd., Phosphorex ( MilliporeSigma ), Evonik Industries AG, Capsulution AG, Abbott Laboratories
Additional Attributes
Dollar sales by product type and application, regional demand trends, competitive landscape, pharmaceutical company preferences for specific technologies, integration with specialized manufacturing channels, innovations in formulation technologies, manufacturing optimization, and clinical outcome enhancement

