Baseline characteristics
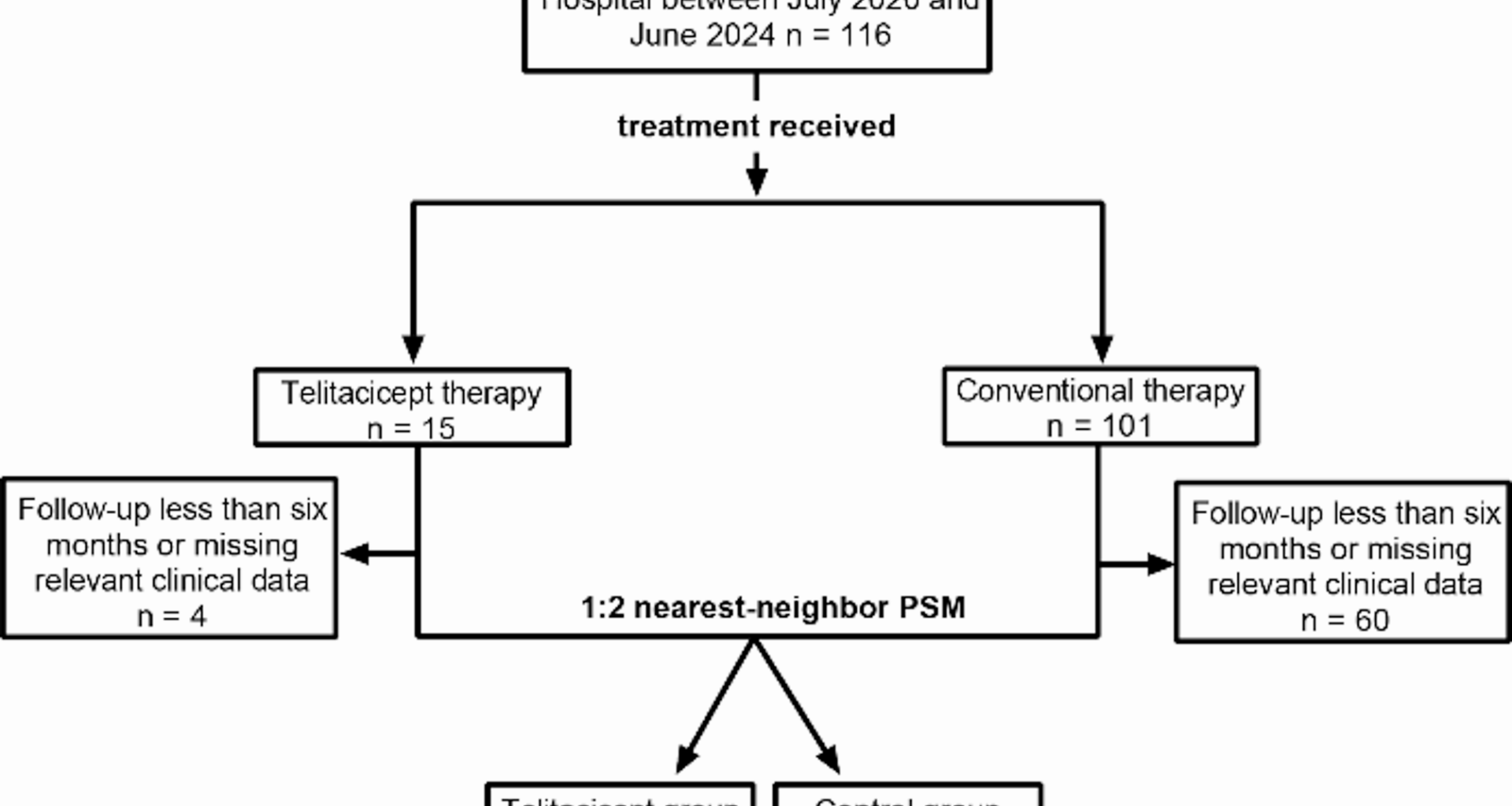
A total of 116 patients with primary IgAN were treated at Xin Hua Hospital between July 2023 and June 2024. After excluding 64 patients due to incomplete data or loss to follow-up, 52 patients were remained, comprising 11 patients in the telitacicept group and 41 in the control group. Baseline demographic and laboratory characteristics before PSM are shown in Supplementary Table 1. Before PSM, patients in the telitacicept group were significantly younger than those in the control group [33.00 (25.00–46.00) vs. 42.00 (37.50–60.00) years, P = 0.030]. To minimize potential confounding effects, PSM was performed using variables including sex, age, BMI, disease duration, 24-hour urinary protein, and serum creatinine. After PSM, the telitacicept group comprised 11 patients, and the control group comprised 22 patients. The patient inclusion process is depicted in Fig. 1. In the telitacicept group, a total of seven females (63.63%) were included. The mean age at disease onset was 35.91 years, with a broad range from 16 to 61 years and the mean duration of disease prior to treatment was 30 months. The median 24-hour urinary protein level was 1244.70 mg. All patients in both groups underwent renal biopsy and were scored according to the Oxford Classification, except for one in the control group, which could not be scored due to an insufficient number of glomeruli obtained during the biopsy, and one in the control group whose detailed biopsy report was missing [16]. Baseline comparisons between telitacicept and control groups are shown in Table 1. No significant statistical differences were observed in demographic and basic clinical characteristics.
The patient inclusion process. PSM, propensity score matching
Table 1 Detailed demographic and baseline clinical characteristics after PSMTreatment pattern
Six patients in the telitacicept group and five in the control group had previously received immunosuppressive therapy, and underwent retreatment, while the remaining patients were treatment-naïve. By the end of the observation, patients in the telitacicept group had received an average of 36 weeks of telitacicept treatment. The patterns of combination medication in the two groups are shown in Table 2. At the initiation of telitacicept therapy, three (27.27%) patients were concurrently using moderate or low doses of corticosteroids, while two (18.18%) patients were receiving MMF. Unlike the phase II trial, the initial dose of telitacicept in our cohort was 160 mg per week [9]. Following disease remission, eight (72.73%) patients had their dosage reduced to 80 mg per week under the guidance of physician. In the control group, 20 patients (90.91%) received corticosteroids with a mean initial prednisone-equivalent dose of 31.25 mg and a mean treatment duration of 8.05 months. In the telitacicept group, 3 patients (27.27%) received corticosteroids with a mean initial prednisone-equivalent dose of 23.33 mg and a mean treatment duration of 3.33 months. All patients adhered to standard treatment protocols for blood pressure management.
Table 2 Combination medicationEffectiveness analysis
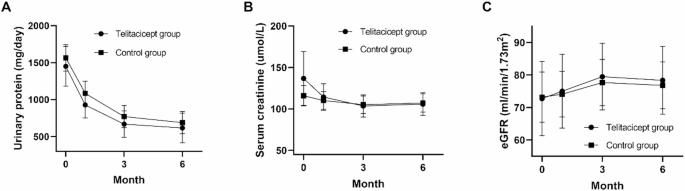
All participants underwent a 6-month clinical follow-up period. Continuous observation was conducted (Fig. 2). Both the telitacicept and control groups showed significant reductions in 24-hour proteinuria from baseline at 3 and 6 months. In the telitacicept group, mean reductions were 779.25 mg/day at 3 months (P = 0.013) and 831.06 mg/day at 6 months (P = 0.006). In the control group, mean reductions were 794.55 mg/day at 3 months (P = 0.004) and 877.06 mg/day at 6 months (P < 0.001). At 6 months, there was no statistically significant difference in 24-hour urinary protein between the telitacicept and control groups [381.68 (182.52–990.00) mg/day vs. 480.15 (185.28–905.15) mg/day, P = 0.567]. No significant differences were observed in creatinine [91.00 (75.00–130.00) umol/L vs. 106.00 (65.00–121.50) umol/L, P = 0.879] or eGFR (78.34 ± 34.52 ml/min/1.73m2 vs. 76.79 ± 33.88 ml/min/1.73m2, P = 0.903) between two groups.
At 3 months, the telitacicept group achieved a complete remission rate of 36.36% and a partial remission rate of 36.36%. Similarly, the control group exhibited a complete remission rate of 31.82% and a partial remission rate of 36.36%. By 6 months, the complete remission rates increased to 54.55% in the telitacicept group and 36.36% in the control group (P = 0.459), with overall remission rates of 72.73% and 77.27% (P = 1.000), respectively. At the final follow-up, only one patient in the telitacicept group experienced a relapse after 8-month treatment. The patient continued telitacicept therapy, which led to subsequent disease remission.
Continuous observation. (A) 24-hour urinary protein; (B) Serum creatinine; (C) eGFR. Error bars indicate standard errors. eGFR, estimated Glomerular Filtration Rate
Discontinuation and safety
Of the 11 IgAN patients treated with telitacicept, four (36.36%) continued treatment until the end of the follow-up period, while seven (63.64%) discontinued telitacicept after a median treatment duration of 34.26 weeks. Five patients discontinued due to complete remission and two due to insufficient efficacy, and all seven were included in the 6-month evaluation. The most commonly reported adverse reactions were injection site pain, redness, or itching, without signs of local infection. These symptoms were reported by five patients and generally resolved spontaneously within two to three days. Two patients had upper respiratory infections during treatment. One patient paused telitacicept due to an upper respiratory tract infection, but resumed treatment successfully after recovery, while the other did not discontinue treatment.
Sensitivity analysis
The standardized mean differences (SMD) for all covariates before and after matching were presented in Supplementary Table 2. After matching, most variables achieved good balance (absolute SMD < 0.2), but a few variables, including age, diabetes proportion, albumin, and IgA, remained imbalanced. To assess the robustness, a sensitivity analysis was performed. Covariates that remained imbalanced after PSM (absolute SMD > 0.2) were included as additional adjustment factors in multivariable regression models. Using this adjusted model, we re-analyzed the overall and complete remission rates at 6 months in patients with IgAN. The results showed that telitacicept treatment was not significantly associated with either overall remission (OR = 3.88, 95% CI: 0.39–38.55, P = 0.247) or complete remission (OR = 1.28, 95% CI: 0.19–8.55, P = 0.796) at 6 months, indicating that the conclusions of the original matched analysis were robust.