Jameela Jamil slams Serena Williams for weight loss drug ad
Serena Williams is under fire after a partnership promoting GLP-1 weight-loss drugs, with actor and activist Jameela Jamil leading the backlash.
unbranded – Entertainment
Liz just wanted to lose the baby weight.
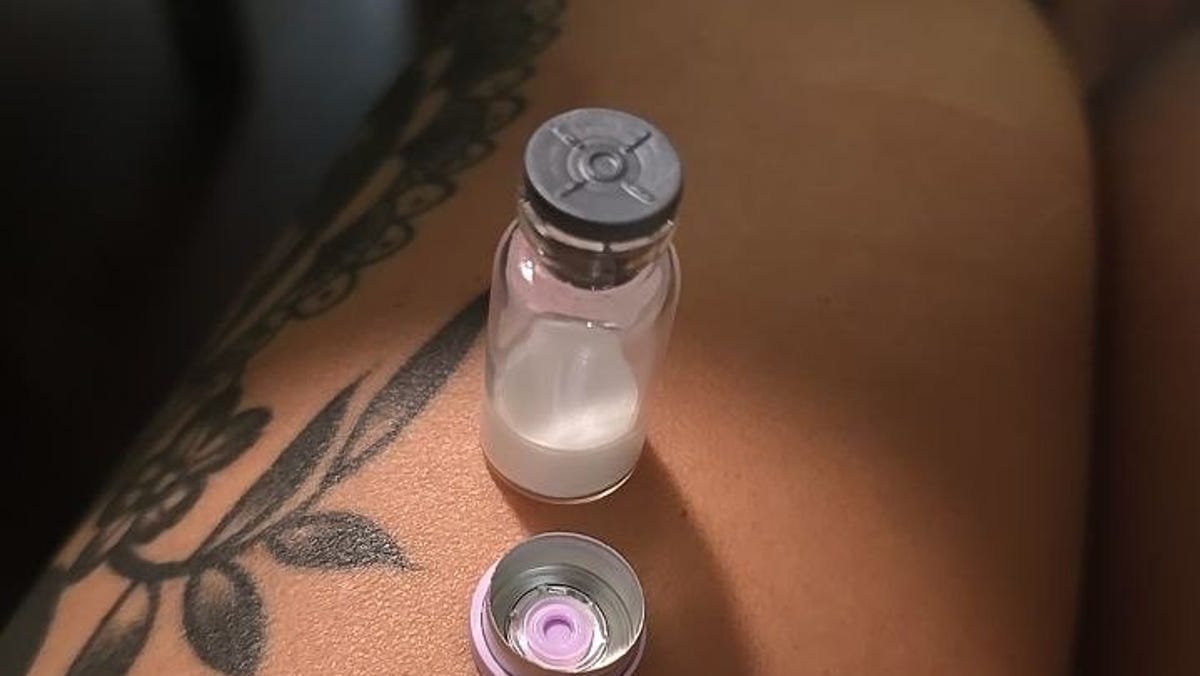
Like so many other moms, the 33-year-old was struggling to fit into her clothes postpartum. She considered compounded versions of weight-loss drugs like Ozempic from online pharmacies but was deterred by the cost. Then, she stumbled upon videos of TikTokers saying these expensive drugs could be made cheaply at home. That was her introduction into the world of “gray GLP-1s.”
“I started in the spring,” she explains of the drugs she prepares in her own kitchen.
Liz, who asked that her last name be withheld due to legal concerns surrounding the at-home mixing process, is among a growing crop of Americans acquiring research-grade peptides and transforming them into a GLP-1-like drug amid affordability and access barriers facing weight loss medications like Ozempic. The process is casually referred to as “gray GLP-1s” or just “gray.”
The popularity of “gray” has medical and legal experts concerned. “Gray GLP-1s” are not approved by the U.S. Food and Drug Administration and purchasing research-grade peptides for human use is illegal on state and federal levels, according to Courtney Sullivan, an Arizona-based attorney who works in drug regulations. But “gray” suppliers promise an attractive way to shed weight and feel better for less.
“The market is wild right now, and these illegal distributors are profiting hand over fist,” Sullivan says. “There is no regulation. There is no enforcement, and there is no safety verification for these products.”
The FDA recently published updated guidance for compounded GLP-1 medications, but more explicit action and enforcement against “gray” is needed: “The only way to know that you are getting a legitimate and safe product is to source it from a state-licensed manufacturer or compounder,” Sullivan says. “These are black market illegal drugs. Calling them anything less conveys some sense of legitimacy that simply doesn’t exist.”
‘Grasping at straws’
Affordability and access drive “gray,” says Sabina Hemmi, founder of GLP Winner, a consumer watchdog. Some microdose the at-home concoctions as a cost-effective maintenance regimen. Others use these mixes amid restock delays from name brands. Many like Liz go “gray” because their base weight or medical history excludes them from insurance coverage.
“It was going to be $300 to $400 a month,” Liz says. Spending about $100 for over 10 months of medication was worth the risk, she says.
Some Americans see options outright disappear. Lindsey Landers, an advanced practice nurse in Kentucky, stopped prescribing compounded GLP-1s from a licensed pharmacy after an August 2025 cease and desist from a drug manufacturer.
Landers continues prescribing brand name GLP-1s to patients who can afford them, but some were forced to make tough choices.
“I have patients who have lost up to 125 pounds and feel like they have gotten their life back,” Landers says. “(My patients) are grasping at straws and willing to attempt to purchase these medications from any accessible source, including illegal internet providers. I service a small community with only six stop lights. Access to care here is a very real concern.”
Why ‘gray’ is a problem
These illegal drugs have medical risks, Sullivan says. Some “gray” products could be defunct, leaving critical health needs unresolved, while others could contain contaminants with dangerous long-term effects.
“These patients are at risk when using these untested and illegal products,” Landers echoes.
Another concern is safety. “Gray” users navigate encrypted chats, heed non-medical advice and use obscure websites that constantly disappear, Hemmi says. Many of the vendors are foreign, requiring payment in cryptocurrencies.
Misinformation is rampant, too. Many take the “research” label on peptides for granted, Sullivan says.
“Research-use only can be a good thing when its actually done in a research environment,” Sullivan says. “These companies are going to tell you things that help solidify their legitimacy.”
Experts say more regulation is critical for patient safety. As “gray” goes mainstream, its users fear a crackdown.
Especially for people who need GLP-1s for chronic health issues, Liz says she understands the panic and wishes there was a cheaper, regulated option.
“It’s not the reality in America right now to be able to afford an extra $400 a month,” Liz says. “Why can’t we have an affordable option?”