Biograph partners with Caristo Diagnostics to bring CaRi-Heart to the US and open preventive pathways in cardiology.
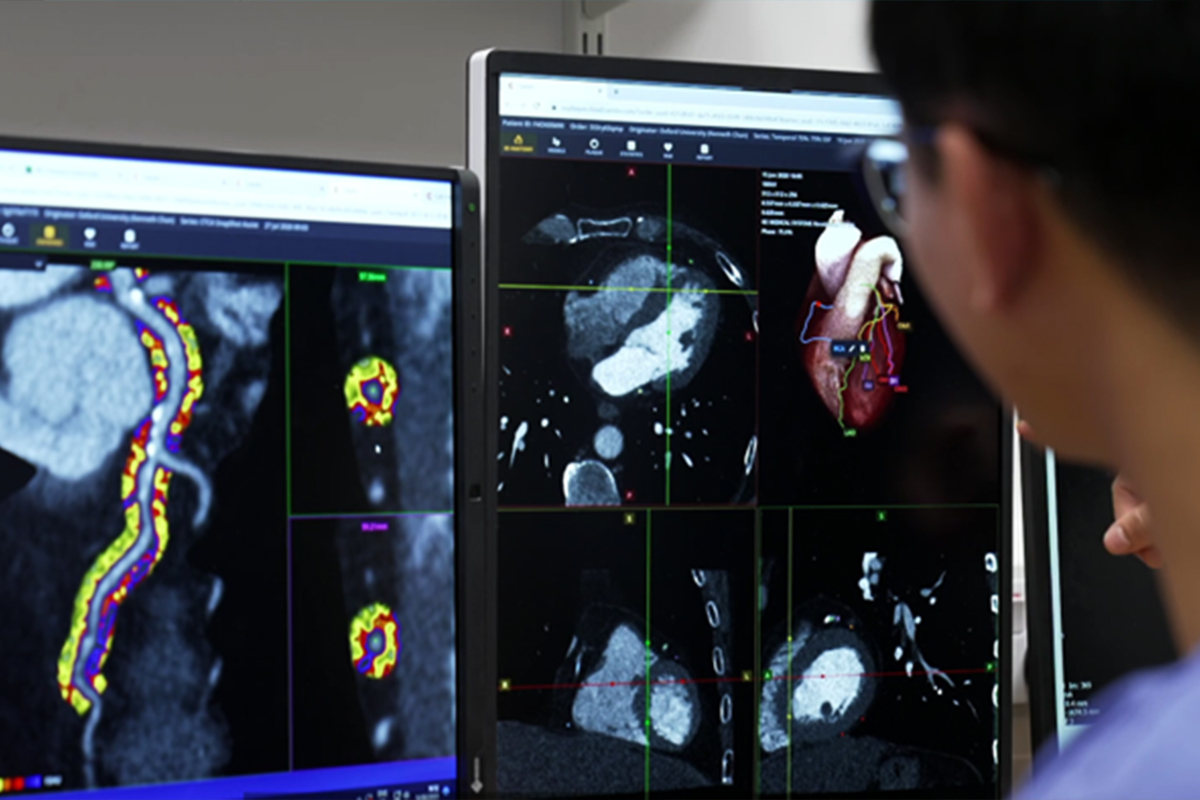
Cardiovascular disease remains the leading global cause of death, and much of today’s prevention still waits for plaque to appear before action is taken. Caristo Diagnostics’ CaRi-Heart technology, already in use across Europe, detects inflammation around the coronary arteries from cardiac CT scans – a shift from plaque-first to inflammation-first models that promises earlier and more precise risk stratification.
Now Biograph, a preventive health and diagnostics clinic in the US, is partnering with Caristo to run the first US study of the technology in asymptomatic patients. The study will begin this month, initially open to 100 of Biograph’s Black Tier members, with participants offered access to both CaRi-Heart and Caristo’s FDA-cleared CaRi-Plaque platform.
Longevity.Technology: Moving from a plaque-first to an inflammation-first model could reshape preventive cardiology, catching risk before structural damage occurs rather than after. With the United States already home to hundreds of longevity clinics – orders of magnitude more than Europe – the environment is primed for earlier adoption, especially among asymptomatic patients seeking proactive care. The implications extend beyond cardiology; if inflammation proves to be the common thread linking cardiovascular, metabolic and neurodegenerative conditions, then its measurement could become a cornerstone of healthspan medicine. Yet the key questions remain: how quickly can such a model move from elite clinics to routine practice, and will regulators and payers be willing to adapt pathways designed for plaque to accommodate inflammation? To find out more, we sat down with Caristo CEO Frank Cheng.
Cheng is clear that inflammation-first detection changes the preventive cardiology equation. “CaRi-Heart clearly moves us closer to being able to intervene before irreversible plaque damage occurs, and even before plaque formation begins,” he told us.
Frank Cheng is the CEO of Caristo Diagnostics.
Cheng highlights a key difference between Europe and the US. “CaRi-Heart analyzes a cardiac CT scan, and in the US, those are typically performed on patients complaining of chest pain. For those patients, we expect significant uptake of CaRi-Heart nationwide once FDA clearance is secured.” But he also sees opportunity beyond symptomatic patients: “One big difference between Europe and the US is the orders of magnitude greater number of longevity clinics in the US, which serve mainly asymptomatic patients. Longevity.Technology estimated there are over 800 longevity clinics in the US, whereas the UK has just 25 and Switzerland, which is a global hub for longevity patients, has 80. The sheer number and growth of longevity clinics here tells us that broader use of CaRi-Heart among asymptomatic patients is not an insurmountable challenge.”
Access is already widening, Cheng says, pointing to momentum both within and beyond the longevity space. “I wouldn’t be surprised if Biograph’s Black Tier offering for Caristo’s solution expands as more of their members hear about it and want to take the test themselves,” he explains.
“Clinically speaking, everyone in a longevity setting can benefit from CaRi-Heart,” Cheng explains adding that tens of healthcare organizations in the UK – ranging from concierge medicine clinics to general cardiology practices and NHS trusts – already use CaRi-Heart as part of their diagnostic toolkit.
The study uses cardiac CT to detect coronary inflammation and plaque in asymptomatic patients, aiming to prevent heart disease before it develops.
And Caristo’s eye is on expansion. “In the US, we will soon be announcing three new customers in addition to Biograph – a second longevity clinic in the West, a large imaging center in the Midwest, and a hospital in the South,” he says. “All of these customers are adopting our CaRi-Plaque solution, which received FDA clearance earlier this year, as well as using CaRi-Heart for research.”
Cheng believes adoption will accelerate quickly once FDA clearance is secured, pointing to evidence already in place. “The 2024 study of 40,000 patients published in The Lancet showed that the predictive power of our FAI-Score is very strong,” he says, adding that CaRi-Heart can determine low, medium and high risk levels for a cardiovascular event over the next 10 years even among people with zero or minimal plaque who would normally be signed off and sent home. “That clinical confirmation is all providers need to be able to say: ‘Great, let’s start using this.’”
Caristo’s AI platform visualises inflammation and plaque to guide prevention.
Cheng notes that the financial case matters as much as the clinical one. “Payers need more than clinical evidence – they want assurance that the technology is also economical,” he says. “And there, too, we already have published data last year in the European Heart Journal – Quality of Care and Clinical Outcomes that shows CaRi-Heart can be economically implemented across the NHS and in other settings.”
The confidence is palpable. “Once FDA clearance is secured, we’re confident that CaRi-Heart adoption will happen very quickly,” Cheng concludes. “Americans already can’t wait to use it; they’re traveling to Europe to take this test.”
Images courtesy of Caristo Diagnostics