Elements of this image of Mars furnished by NASA. (Image by Artsiom P on Shutterstock)
In A Nutshell
A 15-year mystery signal on Mars has finally been identified as ferric hydroxysulfate.
The mineral forms only when iron sulfates are heated above 100 °C with oxygen present.
It was found at Juventae Plateau (linked to volcanic heat) and Aram Chaos (linked to hydrothermal activity).
The discovery shows Mars stayed geologically active long after its early catastrophic events.
Future missions may reveal ferric hydroxysulfate in other sulfate-rich Martian terrains.
AMHERST, Mass. — For more than a decade, a peculiar mineral scattered across Mars has puzzled planetary scientists. Its unique light pattern, known as a spectral signature, didn’t match any known mineral on Earth or elsewhere in the solar system. Now, researchers have finally identified the substance: ferric hydroxysulfate, an iron-based compound that forms when other iron minerals are heated above 100 degrees Celsius in the presence of oxygen.
The breakthrough came through laboratory experiments where scientists heated various iron sulfate minerals under controlled conditions. When heated, these minerals transformed into ferric hydroxysulfate, producing the exact spectral fingerprint observed on Mars by NASA’s orbiting spectrometer.
The mineral appears at two major Martian locations: the plateau above Juventae Chasma and within Aram Chaos, both regions known for their layered rocks rich in sulfate deposits.
How the Mars Mineral Forms
Ferric hydroxysulfate doesn’t form through cold, wet processes like most Mars sulfates. Instead, it requires heat and oxygen. In the lab, scientists heated samples of hydrated iron sulfates to at least 100 degrees Celsius and watched as the iron atoms changed from one form (ferrous) to another (ferric) while releasing hydrogen.
Tests showed how temperature affects the process. Heating samples at 100 degrees Celsius for six days created ferric hydroxysulfate, though higher temperatures sped things up. At 50 degrees Celsius, nothing happened even after seven days, proving that heat is essential.
The mineral’s structure features iron atoms connected by hydroxyl groups in a chain-like pattern. This arrangement creates distinctive spectral features at specific wavelengths, including the 2.236-micrometer signal that first caught researchers’ attention in 2009.
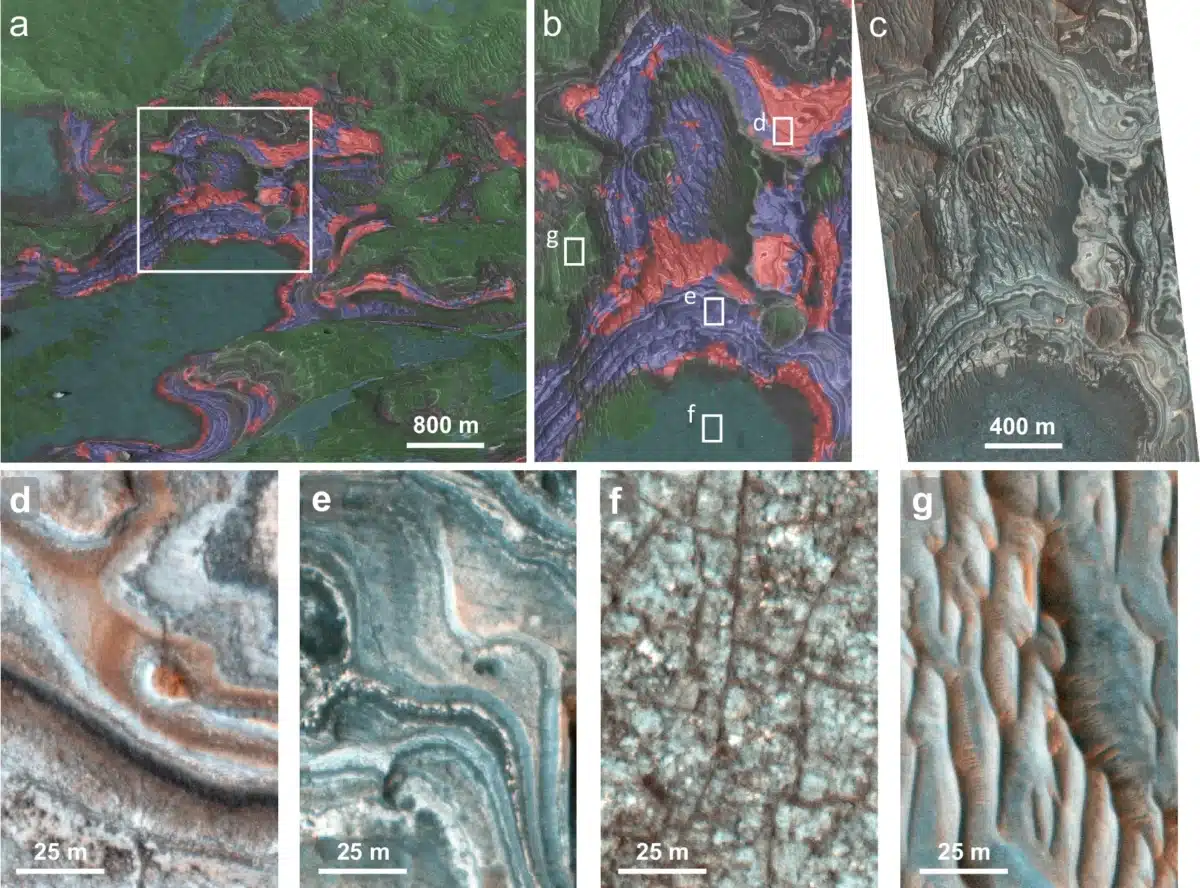
Volcanic Heat at Juventae Plateau, Underground Heat at Aram Chaos
At Juventae Plateau, ferric hydroxysulfate appears in thin layers sandwiched between volcanic rocks and other sulfate deposits. Researchers propose that hot lava or volcanic ash could have covered existing sulfate deposits, heating them enough to trigger the transformation. Some ferric hydroxysulfate also appears beneath the sulfate layers, possibly heated by warm volcanic material below.
Aram Chaos presents a different picture. There, ferric hydroxysulfate sits at the base of sulfate deposits, just above bedrock. The region formed roughly 3 billion years ago when groundwater burst through the surface, causing catastrophic flooding and ground collapse. Later water activity created layered sediments that became sulfates through evaporation. Hydrothermal systems, or underground hot water heated by geologic activity, likely warmed these deposits from below, converting some sulfates into ferric hydroxysulfate.
The different scenarios at each location show Mars had an active and varied geological past. Both sites reveal heating events that happened after the initial sulfate deposits formed, suggesting the planet likely stayed geologically active longer than previously thought.
Eliminating Other Candidates
Identifying the mineral required comparing hundreds of laboratory samples against Mars data. Scientists tested clay minerals, gypsum, and iron sulfate compounds, but none matched. Even combinations of minerals failed to explain what satellites were seeing.
The answer came from heating experiments. When scientists heated szomolnokite (an iron sulfate mineral) to 250 degrees Celsius for 22 hours, the result showed spectral features nearly identical to Mars observations. X-ray diffraction confirmed the heated material was pure ferric hydroxysulfate.
Improved imaging techniques also played a role. New methods for processing satellite data removed atmospheric distortion and noise, revealing previously hidden outcrops. At Aram Chaos, these improvements let researchers identify clear patches of ferric hydroxysulfate, providing cleaner data than the thinner, mixed units at Juventae Plateau.
Mars Stayed Active Longer Than Expected
Finding ferric hydroxysulfate helps refine Mars’ geological timeline. The mineral likely formed less than 3 billion years ago, during a period called the Amazonian. This makes it one of the more recent chemical changes to the Martian surface, lining up with evidence of volcanic and hydrothermal activity that persisted long after the massive events that carved Valles Marineris and the surrounding terrain.
Other sulfate-rich regions on Mars may contain additional ferric hydroxysulfate deposits waiting to be confirmed. One earlier study reported a similar signal in Aureum Chaos, west of Aram Chaos, though researchers at the time interpreted it as a different mineral.
What began in 2009 as an unexplained spectral feature has become a window into Mars’ active past. Future missions may reveal whether ferric hydroxysulfate is more widespread than currently known, offering new insights into how heat and water continued to reshape the Red Planet long after many scientists thought it had gone quiet.
Paper Summary
Methodology
Researchers created ferric hydroxysulfate by heating hydrated iron sulfate minerals at temperatures from 100 to 300 degrees Celsius under controlled conditions. They synthesized starting materials through dehydration processes, then measured how light reflected off samples across a wide range of wavelengths using facilities at Brown University, the German Aerospace Center in Berlin, and the University of Grenoble. X-ray diffraction confirmed what minerals were present. Experiments in sealed containers with nitrogen or air showed oxygen is required for the transformation. For Mars observations, the team analyzed satellite images using advanced techniques to map mineral distributions at Juventae Plateau and Aram Chaos.
Results
Heating samples at 200 degrees Celsius for 26 hours produced pure ferric hydroxysulfate. Lower temperatures worked more slowly: samples at 100 degrees Celsius for six days yielded 81 percent ferric hydroxysulfate, while others required eight days to reach 27 percent conversion. No transformation occurred at 50 degrees Celsius even after seven days. Mars satellite data showed the characteristic 2.236-micrometer signal at both study sites. At Juventae Plateau, ferric hydroxysulfate occurs in thin layers mixed with other sulfates, positioned above and sometimes below other sulfate units. At Aram Chaos, ferric hydroxysulfate patches appear next to and beneath different sulfate minerals. Experiments confirmed the reaction requires oxygen.
Limitations
The study focused on two Martian locations where the unusual light signal is most obvious, so ferric hydroxysulfate may exist elsewhere but hasn’t been characterized yet. At Juventae Plateau, the thin ferric hydroxysulfate units span only 40-50 meters, making measurements likely include mixtures with adjacent sulfates. Determining exact formation temperatures on Mars is difficult because laboratory experiments used hours to days, while Martian processes could have operated over much longer timescales at potentially lower temperatures. The study couldn’t definitively determine whether heat sources were lava flows, volcanic ash, or underground hot water, though the rock layers provide clues. Small variations in the signal position suggest compositional differences that need further study. The research didn’t examine whether ferric hydroxysulfate remains stable under current Martian conditions.
Funding and Disclosures
Support came from NASA grants, Austrian Science Fund, Swedish Research Council, NASA postdoctoral fellowships, and EU Europlanet program. The Reflectance Experiment Laboratory is supported by NASA. The authors declare no competing interests.
Publication Information
Bishop, J.L., Meusburger, J.M., Weitz, C.M., Parente, M., Gross, C., Talla, D., Saranathan, A.M., Itoh, Y., Gruendler, M.R.D., Howells, A.E.G., Yeşilbaş, M., Hiroi, T., Schmitt, B., Maturilli, A., Al-Samir, M., Bristow, T.F., Lafuente, B., & Wildner, M. (2025). “Characterization of ferric hydroxysulfate on Mars and implications of the geochemical environment supporting its formation,” is published in Nature Communications, 16, 7020, August 5, 2025. DOI: 10.1038/s41467-025-61801-2


