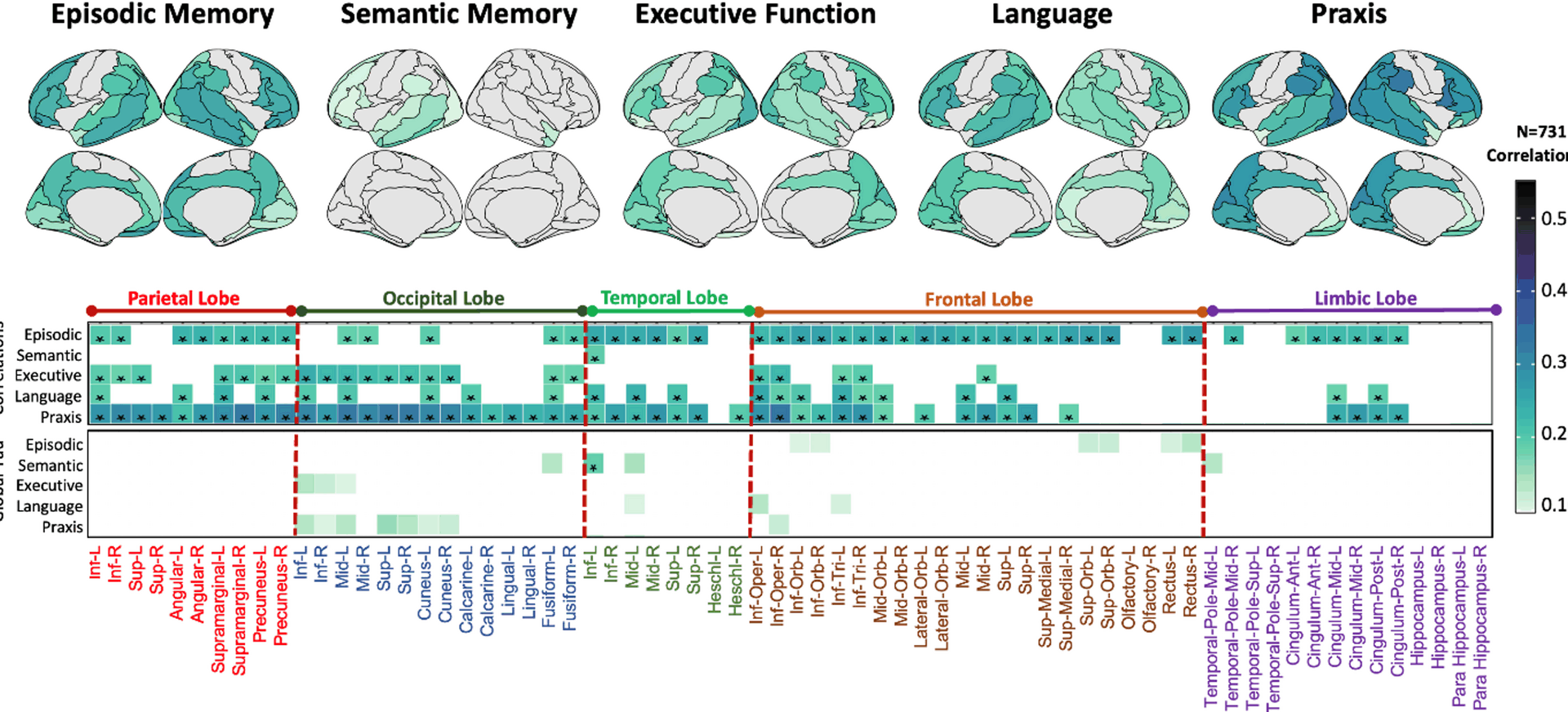
Our study corroborates the prognostic value of tau-PET regional distribution on cognitive decline in several domains, in a clinical trial cohort patients with MCI-to-mild dementia. Differential tau-PET patterns are associated with domain-specific cognitive performance and decline in symptomatic amyloid-positive patients, as shown by both regional correlation and voxel-wise regression analyses. Specifically, higher tau-PET SUVR in an extensive regional pattern encompassing fronto-temporal and parietal regions was predictive of faster decline in episodic memory. Higher tau-PET uptake in the left anterior temporal lobe related to worse semantic memory, while a more extensive and asymmetric tau-PET uptake pattern in (L > R) fronto-temporal and parietal regions and (R > L) fronto-parietal and occipital regions related to faster decline in language and praxis, respectively. Executive decline showed small inter-individual variability, leading to limited association patterns. Region-based analyses highlighted similar tau-PET uptake patterns as predictive of domain-specific decline.
Previous cross-sectional studies on a similar but smaller cohort showed similar tau-PET uptake patterns associated with cross-sectional domain-specific impairment [8, 9]. Thus, the current study replicates and extends previous evidence. Beyond cross-sectional studies, only a limited number of observational and smaller studies have shown associations between regional patterns of tau-PET and cognitive decline in patients on the spectrum of AD. In 152 individuals with CDR ≤ 0.5, with only a small portion of positive amyloid PET scans, retrospective longitudinal analyses indicated that tau-PET had the largest predictive value on episodic memory and executive functioning decline, as compared to amyloid-PET and structural MRI [21]. Moving from pre-clinical to dementia stages on the AD spectrum, in a small cohort (n = 36) of amyloid-positive patients with AD, differential voxel-wise patterns of tau-PET at baseline were found to be strongly associated with subsequent cognitive decline in memory, executive and instrumental functions, while no associations were found with baseline amyloid load and regional cortical atrophy [22]. In a larger cohort of amyloid-positive individuals (n = 131) from the ADNI study, widespread associations between Flortaucipir PET patterns and cognitive decline were found in domain-related regions, as compared to limited cortical thickness-cognition associations [23]. Another ADNI-based study on 140 amyloid-positive individuals confirmed the previous results, reporting that cognitive domain-specific tau-PET outperforms amyloid-PET and structural MRI, but also global and temporal lobe tau-PET, for predicting future cognitive decline in episodic memory, language, executive functioning, and visuospatial abilities [24]. Overall, these observational studies indicated that domain-specific cognitive decline in patients with AD reflects underlying tau progression into associated brain circuits, and spatial patterns of tau-PET may have better sensitivity to capture differential decline than MRI-derived measures.
Our study complements and expands on this series of reports. Specifically, our results point to similar tau-cognition spatial relationships in a larger and more diverse trial-based cohort of amyloid-positive patients with a clinical diagnosis of MCI or mild AD dementia. We focused on early stages of the disease as this may be the most efficacious time window to intervene with disease-modifying therapies, and prognostic biomarkers may be the most appropriate tools to stratify patients at these early stages of disease. Notably, our results showed that the observed asymmetry and spatial differences in the prognostic value of tau-PET for cognitive decline are more pronounced than in cross-sectional correlation patterns with cognitive domains. This aligns with recent evidence suggesting that asymmetric tau deposition is associated with earlier disease onset, greater pathological burden, and more rapid cognitive deterioration compared to symmetric patterns [25, 26]. In addition, our results identify overlapping patters of tau-PET in correlation analyses with episodic memory and praxis performance. This overlap is consistent with evidence that tau pathology in regions such as frontal and parietal cortices is associated with decline across multiple cognitive domains, reflecting the interconnected nature of neural networks supporting memory and praxis. However, episodic memory deficits were related to tau-PET in temporal regions more extensively than praxis, while the latter was associated with a tau pattern involving posterior regions, including occipital cortex, that were not part of the episodic memory related patterns (Figs. 2 and 5). These findings highlight the importance of considering not only the overall burden but also differential spatial distributions and the lateralization of tau pathology when assessing prognosis and tailoring interventions in AD.
Across domains, we observed overlapping regions where tau-PET uptake, grey matter volume, and cognition were significantly associated. This convergence, particularly in temporoparietal and medial temporal areas, highlights the close interplay between tau pathology, neurodegeneration, and cognitive decline. Such overlap supports the hypothesis that regional tau accumulation may drive local atrophy, which in turn contributes to domain-specific cognitive deficits [27]. The associations between baseline gray matter volume and domain-specific cognitive decline, while showing some overlap, were less extensive and strong as compared to the associations between baseline tau-PET and domain-specific cognitive decline. This suggests that tau-PET may be a stronger predictor of domain-specific longitudinal cognitive decline than gray matter atrophy; and while structural brain changes are important, they may not capture the full extent of the neurodegenerative processes affecting cognitive functions. This aligns with cross-sectional multimodal imaging studies on the AD spectrum, showing tau-PET uptake in areas without overt neurodegeneration [4, 5, 10, 28]. Tau pathology may locally precede neurodegeneration, as also suggested by significant associations of baseline tau-PET patterns with prospective atrophy as measured by structural MRI [29, 30] and retrospective longitudinal atrophy (years preceding tau-PET) in both cognitively unimpaired individuals and patients with clinical AD [31, 32]. Moreover, our findings suggest that while there are regions where tau-PET signals and gray matter atrophy are both associated with cognitive decline, gray matter atrophy does not mediate the relationship between baseline tau-PET and cognitive decline. This indicates that tau-PET may be a robust and independent predictor of domain-specific cognitive decline. Baseline MRI may also capture more of the normal variation in brain structure, whereas tau-PET uptake is indicative of pathological process. Further research in clinical settings is necessary to confirm these findings and explore potential underlying mechanisms.
Previous studies have described strong associations between baseline tau-PET and cognitive changes over time across the AD clinical spectrum. In head-to-head comparisons, tau-PET binding in temporo-parietal regions outperformed amyloid-PET and structural MRI measures in predicting global cognitive decline [12,13,14], especially in patients at early AD stages [12]. We relied on ADAS-cog as single tests with domain-specific sub-scores, rather than focusing on extensive cognitive batteries, in order to improve prognosis in clinical practice and trials in a feasible and pragmatic way while capturing different domains. Although a single test cannot fully assess patients’ cognitive functioning and identify co-occurring deficits that a more extensive battery might reveal, it still holds value. Corroborating previous results by transitioning from composite scores to crude single-test sub-scores offers multiple advantages. First, administering a single test is quicker and less resource-intensive, making it suitable for settings with time constraints or limited access to testing materials, such as clinics that will soon start implementing disease modifying treatment delivery. Second, shorter testing time minimizes fatigue and stress for patients, which can result in more accurate and reliable performance. Third, a single-test assessment is easier to repeat over time for monitoring changes in cognitive function, such as in longitudinal studies or ongoing treatment assessments, reducing data missingness and subjective variability in multiple-test battery administration.
As compared to previous reports, we also implemented a different approach to quantify cognitive changes over time, utilizing LGCM. This analytic approach offers several advantages over other previously used approaches, such as linear mixed effects models and the analysis of absolute differences for tracking changes over time, and has been successfully implemented in prognostic analysis in dementia [14, 33,34,35]. In particular, LGCM (i) explicitly models individual differences in initial status (intercept) and rate of change (slope), allowing for a detailed understanding of how individuals differ in their growth trajectories; (ii) handles missing data more robustly through full information maximum likelihood estimation, which uses all available data points and provides unbiased parameter estimates under the assumption that data are missing at random; (iii) offers a range of fit indices to assess model fit and compare alternative models, facilitating the identification of the best-fitting growth model over complex growth models [36]. LGCM may be a powerful tool to implement in future AD clinical trials, as it provides a more nuanced, flexible, and robust approach to tracking changes over time compared to linear mixed effects models and the analysis of absolute differences.
Notably, for both cross-sectional and longitudinal analyses in our cohort, after adjusting for global tau-PET uptake and global grey-matter volume, the observed domain-specific associations were spatially limited to core brain regions for each cognitive function. This spatial limitation highlights the importance of regional pathology over global disease burden in driving specific cognitive deficits, and the selective vulnerability of functionally specialized brain networks. Regional localized patterns of tau accumulation predict domain-specific cognitive decline and may inform both the timing and type of interventions. These findings reinforce the value of regional PET assessment and support the use of targeted approaches in both research and clinical interventions. Early detection of regionally restricted tau could enable targeted therapies and tailored clinical management, supporting a personalized medicine approach to delay or mitigate decline in specific cognitive domains.
Our study has some limitations. First, the number of cancellation tests (part of ADAS-Cog13) was not available for participants in the A05 study, limiting our evaluation of executive domain to the AMARANTH and EXPEDITION studies. and we acknowledge that ADAS-Cog13 is able to evaluate more sensitively some cognitive domains (i.e., memory) than others. Additionally, w-scores for the executive domain were not calculated as controls had ADAS-Cog11 scores only available. Second, the control group was not age-matched, as it included participants across a wide age range. Notably, some control participants were younger than 50 years old (n = 15, mean age = 29.0), which may introduce some biases in the representativeness of the cohort. The inclusion of younger individuals could lead to an overestimation of cognitive differences between patients and controls, as younger participants are likely to perform better on cognitive assessments due to age-related advantages in processing speed and memory. Third, our mediation analyses were restricted to evaluating local relationships and did not account for potential distant relationships between tau-PET, gray matter atrophy, and domain-specific cognitive performance/decline. Finally, participants were recruited from expert clinical trial centers under highly controlled conditions, introducing selection bias. This might not accurately represent the broader patient population actually seen in the clinics. Additionally, the study population lacked racial and ethnic diversity, with over 90% of participants being white. The association between tau, neurodegeneration and cognition may vary based on race and ethnicity as proxies for social determinants of health [37]. Other individual factors can also modify the relationship between tau accumulation and cognition, with younger age, female sex, higher education and cortical thickness being related to increased reserve and resistance against the effect of pathology on cognitive performance [38]. Much more work in diverse cohorts is needed to validate our results in population-representative cohorts.
In summary, we showed that differential regional patterns of tau-PET are associated with domain-specific cognitive decline in a large cohort of patients with MCI and early AD dementia. Implementing latent growth curve modelling approaches on single-test longitudinal changes may empower prognosis and outcome prediction. Tau-PET may be a good tool to stratify patients in clinical trials of disease-modifying therapies, with personalized predictions of neurodegeneration and cognitive trajectories, enhancing the chance to identify the best time window and cohort where a given therapy can be most effective.