Our method estimated an average health expenditure on major NCDs across OECD member countries of US$207 million per 100,000 population. The USA had the highest expenditure at US$599 million per 100,000, and (according to our method) musculoskeletal disorders topped the list of NCDs with the highest proportion of total health expenditure across member countries. After pooling health expenditure on NCDs across countries, females had significantly higher expenditure on musculoskeletal disorders, as well as mental and substance use disorders, and neurological conditions. Males had significantly higher expenditure on kidney and urinary diseases, and cancer and other neoplasms. First year of diagnosis represented on average 36.8% of total NCD expenditure, while last year of life expenditure accounted for 2.6%.
OECD member countries were chosen for this study as we assumed that health expenditure in Australia and NZ would be similar in relative pattern to these countries, given similarities in disease burden patterns (i.e. NCDs are responsible for most of the disease burden in high-income countries). However, for the three country-specific studies that we could compare our method to – Norway, Switzerland, and the USA [9,10,11] (Figs. 4 and 5) – the agreement on the percentage of expenditure by disease was often poor.
Comparing between expenditure studies is challenging, due to differences in disease group classifications, health care function expenditure areas, and years that the data was based on – as well as ‘actual’ variation in expenditure by disease between Australia (the base of our method) with other countries. In Norway a large amount of expenditure on long term care is considered health care expenditure – whereas in Australia long term care is generally not considered health expenditure and is excluded from disease expenditure estimates. The Norwegian analysis by Kinge et al. (2023) included service data on general practitioners, physiotherapists and chiropractors, day patient, specialised outpatient, inpatient, prescription drugs, home-based long-term care, and nursing homes [9]. The Australian analysis included expenditure on hospital admitted patients, outpatients and emergency departments, government subsidised medical services (GP, specialist, allied health, medical imaging, and pathology), prescription pharmaceuticals, and dental [13]. Given the differences in how health system expenditure is classified between these countries, the Norwegian study had much higher estimated expenditure for dementia than our study.
The USA analysis by Dieleman et al. (2016) included service data on ambulatory care, inpatient care, pharmaceuticals, emergency care, and nursing facility care [11]. While there were differences in service data used in the USA and Australian analyses, differences in medication costs between the two countries would have likely contributed to differences in cost estimates for diseases like diabetes.
The OECD has undertaken initial comparisons of disease expenditure across member countries. While this used different methods, data sources, and had a limited scope, it found that CVD was estimated to be the highest expenditure group, while in our study musculoskeletal was the highest, followed by cancer and other neoplasms then CVD [12]. Females still accounted for higher expenditure on musculoskeletal disorders, and mental and substance use disorders, while males now accounted for higher expenditure on kidney and urinary diseases, and cancer and other neoplasms [12].
Strengths and limitations
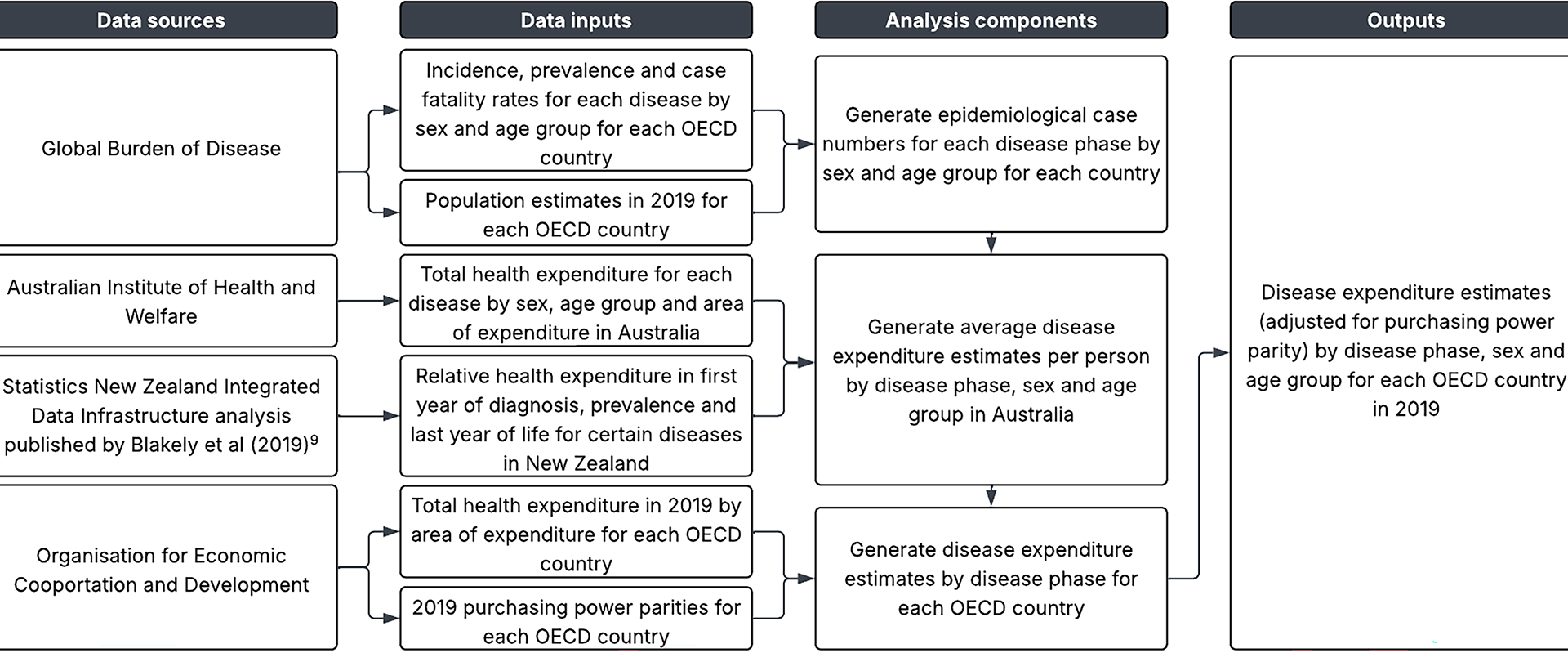
Our study leveraged high quality, comprehensive data from internationally renowned data sources to generate disease expenditure estimates across OECD countries. The GBD is the most comprehensive observational epidemiological study worldwide, while the OECDs online database is the most comprehensive source of comparable statistics on health systems across industrialised countries. The Blakely et al. (2019) analysis utilised linked health data from Statistics NZ, and both that source and the AIHW are known for their comprehensive datasets that capture comorbidity-adjusted costs for each disease and costs to all payers for expenditure areas [8, 13].
There are limitations of this study. Only 73% of recurrent expenditure in Australia was allocated in the AIHWs disease expenditure 2018-19 study [18]. Areas of expenditure not included were over-the-counter pharmaceuticals, other health practitioners, community health, public health, and research remaining unallocated [18]. An assumption of our study is that this missing disease-related expenditure is distributed similarly to the included expenditure (this missing gap being assumed to be another 8% of all health expenditure using the 81% average across OECD countries of national health accounts expenditure on ‘health care functions’ that we assumed equated to the marginal expenditure driven by diseases).
Other key concerns include other OECD countries having different relative expenditure per capita than Australia. For example, the cost and modality (inpatient versus outpatient) of chemotherapy delivery may differ for the same cancer between countries, altering the relative expenditure per capita within cancers. Similarly, the NZ relative costing ratios by disease phase may not be applicable to all OECD countries due to differing medical care and treatment strategies by disease phases, such as approaches to end of life care (expensive treatments versus palliative care).
Australia has a notably higher musculoskeletal disease burden (measured in DALYs by sex and age) than other counties [21]. But estimating musculoskeletal disease expenditure in other OECD countries applied the Australian expenditure per case to each OECD’s prevalence of musculoskeletal disease. Thus the higher musculoskeletal disease expenditure our method estimated in other OECD countries is due to a high expenditure rate per prevalent case in Australia – an area that warrants further research to understand why.
The OECD’s health expenditure accounts may not be capturing all health expenditure within member countries due to within-country limitations on data availability and extent to which data is captured. The GBD estimates of disease prevalence are a function of within country data and smoothing based on similar countries; whether this produces better estimates than ‘just’ using within country disease data is moot. However, for serious bias from GBD estimates to propagate through our method when looking at aggregated diseases (e.g. CVD) would require an over- or under-estimate to apply to most or all the subsidiary diseases in that category.
In this paper, we have not presented uncertainty intervals about estimates – because the AIHW and OECD data do not have uncertainty intervals to propagate through a Monte Carlo analysis. There will be considerable uncertainty about disease expenditure estimates in each OECD country for reasons above through to measurement error in the source data. Such uncertainty is a priority to reduce in future research (next section).
Research and policy implications
This is the first known study to have produced disease expenditure estimates by disease phase, sex, and age group across many countries. These estimates provide governments and researchers with a tool to cautiously use to quantify the economic and health system impacts of NCDs, identifying priority diseases attributing the most burden to national health expenditure, informing the allocation of health system expenditure, and improving health system efficiency. But our estimates are a ‘first attempt’ at comparable cross-country disease expenditure estimation – improvements are required.
Future research should look further into the reasons for the residual variation between health expenditure across countries, between our study and all three of the Norwegian, Swiss and US studies [9,10,11]. On the one hand, progress is needed to make country-level expenditure comparisons less prone to methodological and data differences – meaning any remaining differences are ‘true’ and due to (say) differences in pharmaceutical expenditure, patient care pathways, and any number of other factors. On the other hand, as we gain confidence in what the ‘true’ differences in country-level expenditure are, then predictors of such variation should be identified. For example, private versus public expenditure, greater or lesser focus on new technologies, GDP per capita, per capita pharmaceutical expenditure, and such like. We envisage such predictors being used in predictive algorithms (e.g. machine or ensemble learning) that takes what data we can collate between countries and ‘fills in the gaps’ to give better estimates of expenditure by disease in all countries.