A 27-year-old Male patient presented to our hospital with a 4-year history of left joint pain and discomfort, accompanied by headaches. The pain had gradually intensified over time, impairing his sleep quality. The patient denied any history of specific trauma or surgery.
Physical examination revealed grossly symmetrical facial anatomy, with normal interincisal opening measuring(47 mm) and a linear mandibular excursion pathway(Fig.1). During opening and closing movements, bilateral temporomandibular joint (TMJ) clicking sounds were absent and the patient reported pain in the left TMJ area, failed to precisely localize the pain, and exhibited concurrent pain in the temporal region. There was no tenderness in the joint area, and the bilateral posterior push mandible test was negative when the mouth was half-opened. Palpation of bilateral masticatory muscles (masseter, temporalis, medial pterygoid and lateral pterygoid) revealed no tenderness, and muscle tone was within normal limits. Furthermore, intraoral examination revealed no further abnormalities. Occlusal relationship was unremarkable, with no evidence of dental malocclusion or occlusal interference. Cone beam computed tomography (CBCT) revealed a well-defined hypodense lesion extending from the medial aspect of the left glenoid fossa to the cranial base, containing hyperdense calcific foci within the hypodense matrix. Additionally, hyperdense osseous changes were observed in the temporal bone, glenoid fossa, and posterolateral orbital region. (Fig. 2). Prior to histological characterization of the lesion, a provisional diagnosis of TMJ mass was established, necessitating surgical intervention for therapeutic management and definitive histopathological verification. After confirming that the patient’s physical condition met the surgical requirements, the patient was intubated under general anesthesia through the nose and the TMJ was exposed through the preauricular incision. Partial capsulotomy revealed no discernible anterior disc displacement. Fibrous adhesions were identified between the posterior band and bilaminar zone within the posterior recess of the superior joint space. Concurrently, demineralized bone with fragile consistency was observed at the lateral articular eminence. (Fig 3).Under irrigation, a piezoelectric osteotome harvested a 0.5 × 0.8 × 0.3 cm specimen from the eminence lesion for histopathological examination.Correlation with preoperative imaging localized the pathology to the posterosuperior articular eminence. The superficial periosteum demonstrated no abnormalities. After subperiosteal dissection using a Molt elevator, demineralized bone with friable consistency was encountered, extending toward the middle cranial fossa. Gentle curettage with a bone curette yielded fragmented osseous tissue containing particulate cortical fragments within the matrix. A specimen approximately 1.0 × 1.0 cm was harvested from the glenoid fossa lesion for histopathological analysis. Post-debridement assessment confirmed well-defined margins and preservation of cranial floor cortical integrity.Regarding osseous reconstruction, intraoperative assessment confirmed no discernible disc displacement necessitating repositioning. The residual osseous defect measured approximately 1.2 × 1.3 × 1.5 cm³. Following meticulous hemostasis achieved via electrocautery, the cavity was preserved for secondary bone regeneration without grafting.
Symmetrical bilateral faces(pre-operative)
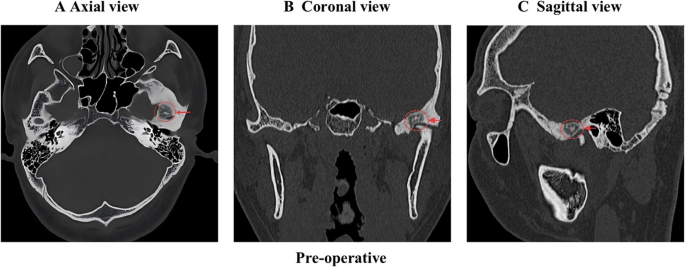
CBCT demonstrated a hypodense lesion within the left temporomandibular joint (TMJ), measuring 8 mm × 11 mm in diameter, accompanied by diffuse sclerosis of the adjacent osseous structures. A Axial view; B Coronal view; C Sagittal view. The red arrow and circle represent the hypodense lesion
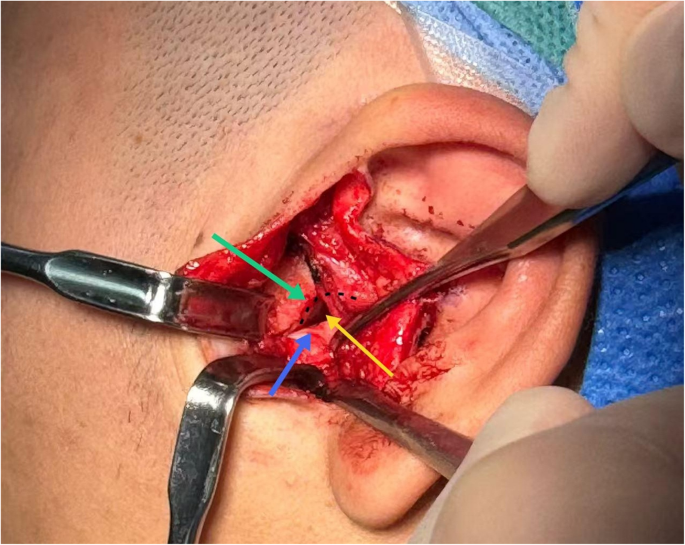
Operative image. The black curve represents the morphology of the glenoid fossa, the green arrow points to the articular eminence, the blue arrow indicates the articular disc, and the yellow arrow denotes the surgical approach
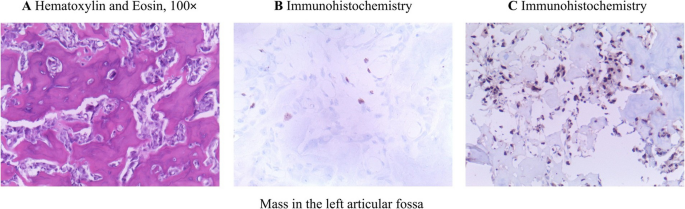
Histopathological analysis of the left articular fossa mass demonstrated extensive interlacing osteoid trabeculae forming a woven bone-like architecture, characterized by irregular morphology and disorganized arrangement. These trabeculae were encircled by a prominent rim of plump, round osteoblasts. Immunohistochemical analysis revealed upregulated SATB2 expression and low Ki-67 expression. SATB2, a transcriptional regulator implicated in osteoblastic differentiation and skeletal development, demonstrates increased activity in this context. The low Ki-67 proliferative index indicates diminished mitotic activity and slower tumor growth kinetics, suggesting a lower likelihood of malignant potential. The histopathological features observed in hematoxylin-eosin staining, corroborated by immunohistochemical findings and clinical history, collectively supported the diagnosis of Osteoid Osteoma (OO) (Fig. 4).
A Micrograph shows a meshwork of interconnected trabeculae of woven bone with prominent osteoblastic rimming in a background of vascularized connective tissue; B Results of immunohistochemistry: Ki-67 Antigen Labeling Index(3%+); C Results of immunohistochemistry: Special AT-rich sequence-binding protein 2 SATB2 (+)
At the one-month postoperative follow-up, the patient demonstrated complete resolution of nocturnal pain and restoration of normal mandibular range of motion. Three-month postoperative clinical imaging demonstrates satisfactory functional and aesthetic outcomes without evidence of complications. (Fig. 5). Three-month follow-up imaging revealed complete eradication of the OO with no evidence of tumor recurrence observed(Fig. 6).
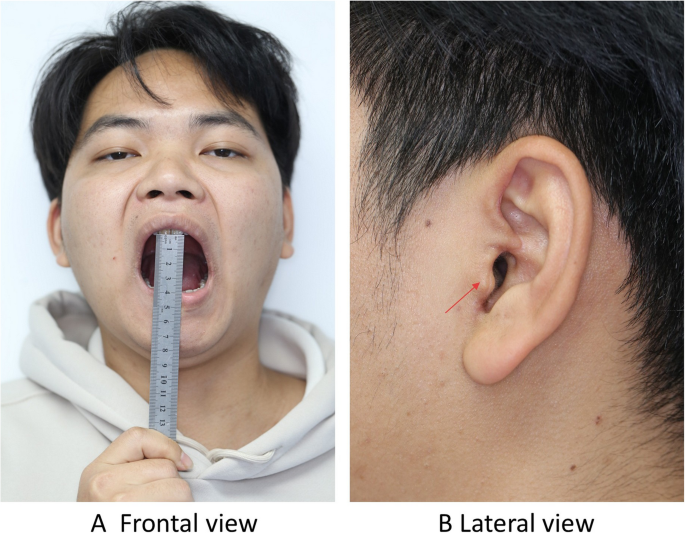
Postoperative frontal photography at the 3-month follow-up demonstrates normal mandibular opening(40 mm) without functional impairment, while the lateral view reveals a well-healed surgical incision with no evidence of hypertrophic scarring or aesthetic compromise. A Frontal view B Lateral view
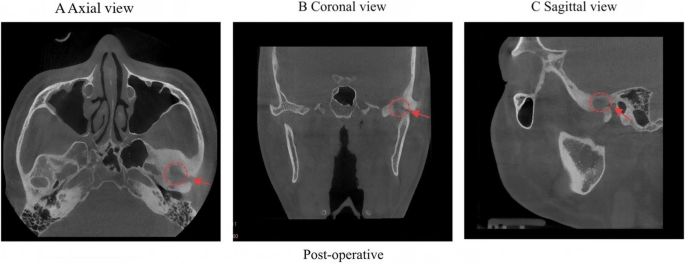
Postoperative CBCT demonstrated complete tumor resection with well-defined margins exhibiting density equivalent to adjacent normal bone tissue. A Axial view B Coronal view C Sagittal view. The red arrow and circle represent the residual surgical defect in TMJ
Discussion and conclusion
Osteoid Osteoma (OO) is a prevalent benign bone neoplasm, more than 50% of cases arising in long bones, predominantly the femur and tibia [6, 7]. However, OO may also develop in periarticular or intra-articular locations, with intra-articular variants accounting for approximately 10% of cases. Compared to long bone involvement, intra-articular occurrences are relatively uncommon, primarily affecting large joints including the knee, hip, shoulder, and elbow [10]. Notably higher incidence rates are observed in the knee and hip joints, frequently associated with anatomical interactions between osseous structures and articular cartilage or adjacent periarticular tissues. Although OO predominantly arises in the diaphysis of long bones, craniofacial involvement may occur, particularly affecting the mandible, maxilla, and temporomandibular joint (TMJ). OOs localized in the maxillofacial region and TMJ account low prevalence in the literature [12,13,14]. The clinical presentation in these craniofacial regions often mimics other oral and maxillofacial pathologies due to anatomical proximity to articular structures and dentoalveolar components [14, 15].
An English-language literature review was performed utilizing the PubMed and ScienceDirect databases, with “Osteoma, Osteoid” designated as the principal search parameter complemented by region-specific terms including “maxillofacial,” “maxilla,” “mandible,” “mandibular,” “TMJ,” “Temporomandibular Joint,” “articular eminence,” “glenoid fossa,” “condyle,” “condylar,” and “jaw.” After the review, 34 reports of 36 cases of OO involving both the jaw and the TMJ were found and selected for this review(since 1951) [11,12,13,14, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45].Through our review, we have found that OO occurring in the TMJ are centered on localized pain, which is mostly characterized by exacerbation at night and during chewing. Moreover, they are often accompanied by functional impairments such as trismus and mandibular deviation, with some cases presenting swelling or joint clicking. Due to the similarity to temporomandibular joint disorders(TMD), OO are prone to being missed in clinical practice and thus deserve attention. The relevant information is summarized in Table 1.
Table 1 Overview of the literature: osteoid osteomas involving the TMJ
OO in long bones typically manifests as persistent dull pain characterized by nocturnal exacerbation with heightened intensity. A diagnostic feature of this pain pattern is its responsiveness to NSAIDs administration [6, 46]. Although localized swelling remains generally mild, the tumor site frequently demonstrates marked tenderness upon palpation, with a subset of patients exhibiting mild periosteal or soft tissue edema in the affected region. The occurrence of OO in the joint, in addition to pain, can also lead to joint movement dysfunction, swelling, and effusion [47].
OO involving the TMJ may exhibit clinical manifestations distinct from extra-articular presentations, featuring persistent nocturnal pain aggravated by masticatory and mandibular opening movements, restricted joint mobility demonstrated through limited jaw excursion, articular stiffness, and compromised mastication, accompanied by abnormal joint acoustics (clicking or crepitus) and localized thermogenesis post-articulation [12]. In the case that we presented, the patient manifested chronic left-sided TMJ pain with concomitant ipsilateral cephalalgia persisting over a four-year disease course.
The pathogenesis of OO remains incompletely elucidated, with multiple hypotheses currently under investigation [48]. Although the precise mechanisms remain incompletely elucidated, current research highlights osteoblast hyperactivity and osteosclerotic reactions as predominant etiological factors. Concurrently, localized inflammatory responses, vascular hyperplasia, and potential genetic contributors participate in the disease progression [4, 49]. In this case, the absence of significant personal medical history, prior comorbidities, familial predisposition, or local mechanical irritation/trauma further underscores the necessity for continued investigation into the multifactorial pathogenesis of OO.
The diagnostic foundation for OO involving the TMJ is started from Physical examination. Physical examination was performed according to the (Diagnostic Criteria for Temporomandibular Disorders) DC/TMD protocol [50]. Standardized palpation with 1 kg/cm² pressure was applied to bilateral masticatory muscles (masseter, temporalis, medial/lateral pterygoids) and TMJ lateral poles/posterior attachments [51]. Mandibular range of motion was measured using a caliper, including maximal unassisted opening, assisted opening, and lateral/protrusive excursions. Joint sounds were assessed via stethoscopic auscultation during dynamic movements. The DC/TMD protocol for evaluating the TMJ and masticatory muscles encompasses medical history assessment, clinical evaluation, imaging studies, and other examinations, thereby establishing a comprehensive evidentiary basis for diagnosis [52].
Following evaluation of the patient’s medical history and physical examination, we documented clinical features inconsistent with prevalent TMD. For example, patients with TMJ osteoarthritis characteristically develop motion-triggered arthralgia and audible joint phenomena including clicking or crepitus [53, 54]. Fibrous dysplasia of the TMJ is typically not associated with significant pain. The condition predominantly manifests as facial contour asymmetry and malocclusion [55, 56]. These deviations from the classic presentation of OO represented clinical red flags, warranting advanced imaging studies and definitive histopathological verification.
Definitive diagnosis of TMJ OO necessitates multidisciplinary synthesis of advanced imaging modalities and histopathological verification to achieve diagnostic certainty. Computed tomography (CT) serves as a critical diagnostic modality, enabling precise delineation of lesion localization, dimensions, and morphological characteristics. It demonstrates significant diagnostic superiority in nidus detection, typically manifesting as well-defined, round or ovoid hypodense lesions within articular regions. These lesions frequently exhibit central calcific foci within the hypodense matrix, with calcification density correlating positively with tumor maturity. Characteristically, the nidus is circumferentially bordered by reactive sclerotic bone formation [57, 58]. Although Magnetic Resonance Imaging (MRI) is not as accurate as CT in showing the nidus, it can offer valuable soft tissue characterization through articular contrast resolution, permitting assessment of peri-lesional structural involvement and its capacity to identify secondary indicators including joint effusions, synovial hypertrophy, and bone marrow edema contributes substantially to diagnostic confirmation and differentiation from other arthropathies [59].
The differential diagnosis of OO proves critical given its clinical overlap with numerous osteoarticular pathologies, particularly due to shared manifestations of localized pain and articular dysfunction [60]. For example, compared with osteoblastoma, OO usually has significant nocturnal pain and has a certain response to NSAIDs, and the pain of some patients can be relieved after taking NSAIDs. However, osteoblastoma pain characteristics lack the characteristic nocturnal exacerbation and NSAIDs responsiveness pathognomonic of OO, demonstrating nonspecific analgesic reaction patterns [46, 61]. On CT imaging, OO typically manifests as a well-circumscribed hypodense lesion containing a central calcific focus within the hypodense matrix, surrounded by reactive sclerotic bone formation, and generally measuring less than 2cm in diameter. In contrast, osteoblastoma typically presents as larger lesions, generally exceeding 2 cm in diameter. These lesions rarely exhibit central calcific foci and predominantly manifest as hypodense areas on imaging [57, 60, 62].
OO of the TMJ, fibrous dysplasia, TMJ osteoarthritis, and chronic osteomyelitis may present with overlapping clinical manifestations including arthralgia and restricted mouth opening. Definitive radiographic differentiation is achievable through characteristic imaging features. Fibrous dysplasia demonstrates diffuse bony heterogeneity with ground-glass opacity or cystic changes, lacking both a discernible nidus and sclerotic rim [63]. TMJ osteoarthritis is characterized by joint space narrowing, condylar osteophytosis, and subchondral sclerosis, without evidence of an isolated hypodense nidus [64]. Chronic osteomyelitis exhibits concurrent osteolytic destruction and sclerotic proliferation, accompanied by increased medullary cavity density, periosteal reaction, and sequestrum formation. These lesions typically display extensive involvement with frequent soft tissue extension, and absence of the classic nidus architecture [63, 65]. This radiographic hallmark provides essential diagnostic insight for clinicians, particularly those in early career stages.
Therapeutic management of OO in the TMJ centers on minimally invasive interventions (such as radiofrequency ablation, arthroscopy, etc.) and surgical resection, aiming to remove the tumor and relieve symptoms [2, 66, 67]. For patients with mild symptoms, NSAIDs can also effectively relieve pain [4].Therapeutic selection necessitates rigorous risk-benefit assessment balancing therapeutic efficacy against procedural morbidity, with particular emphasis on long-term biomechanical sequelae associated with open or percutaneous articular interventions.
OO in the joint is relatively rare, and OO in the TMJ is more likely to be misdiagnosed as TMD. In this article, a case of OO involving the temporal bone and skull was reported. Due to the rarity of the lesion, CBCT was used for imaging analysis to assist in surgical positioning. After the operation, the patient’s morphology and function recovered well, and the histological examination confirmed the diagnosis of OO.