Cutaneous metastases from thyroid cancer are uncommon. They typically arise in the setting of diffuse neoplastic illness. Some studies have reviewed the English literature from 1964 onward and discovered 43 cases of thyroid cancer with skin metastases [8, 9]. Metastasis to the skin occurs because of lymphatic or hematogenous dissemination of the tumor. The most common sources of cutaneous metastases are, in generally accepted order of frequency: breast, melanoma, lung, colon, stomach, upper aerodigestive tract, uterus, and kidney [10]. Thyroid cancer is the most common type of endocrine-related cancer. Spread of thyroid cancer outside the neck (metastases) is rare, occurring in between 1.2% and 13% of patients [11,12,13]. Metastatic sites include cervical lymph nodes, lungs, and bone [14]. Metastases to the brain, breast, liver, kidney, muscle, and skin are rare [15].
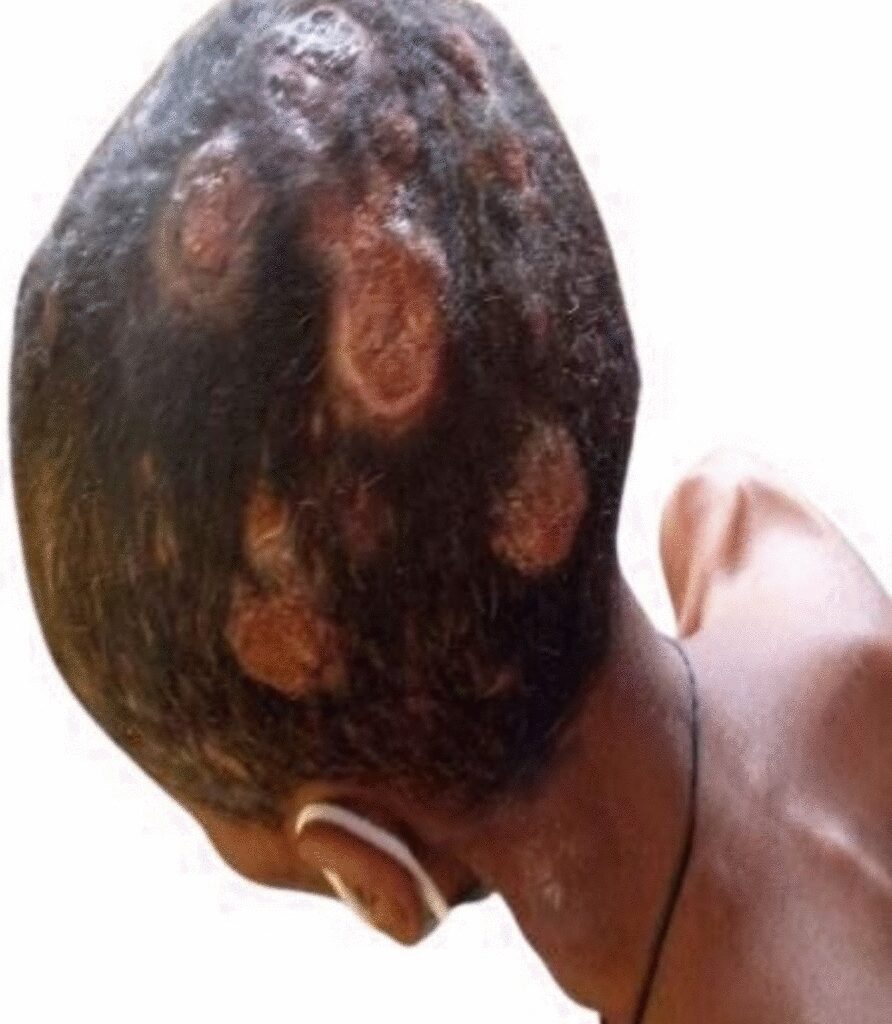
The classic clinical presentation of cutaneous metastasis is a rapid onset of solitary or multiple, asymptomatic, skin-colored, mobile, firm, round or oval nodule(s). The color varies, from skin-colored to pink–red to blue–black. Additional presentations could be erythematous patches that resemble erysipelas or dermatitis (carcinoma erysipeloides), indurated plaques, dermal infiltration causing sclerosis (carcinoma en cuirasses), vascular changes (carcinoma telangiectodes), dermatitis-like appearance of Paget’s disease, pyogenic granuloma-like, reticulated vascular patterns, and Zosteriform.
Diagnosis is based on clinical and pathologic assessment of the skin involved. Histopathologic features help identify the source of the primary tumor. Microscopically, the collection of neoplastic cells, which usually resemble their malignancy of origin, is seen in the dermis and/or subcutaneous tissue. Additional features, such as tumor cells in an “Indian filing” pattern, lymph vascular invasion, necrosis, and a tumor-free “grenz zone,” are also helpful for the diagnosis of metastatic skin lesions. Immunohistochemical staining is often required to achieve the correct diagnosis in a presumed poorly differentiated carcinoma.
Treatment requires a multidisciplinary approach, including medical and surgical oncologists, radiation oncologists, and mental health care providers. Treatment follows the regimen appropriate for the primary metastatic malignancy. Removal of skin lesions by simple excision may enhance the patient’s quality of life but has little effect on the outcome that is dictated by the primary cancer. For functional, palliative, and cosmetic outcomes, local treatment is indicated. Local treatment options include imiquimod cream, liquid nitrogen cryotherapy, photodynamic therapy, excision, carbon dioxide laser therapy, pulsed dye laser therapy, topical and intralesional chemotherapy, and cytokines. When malodorous, topical metronidazole solution can be applied via cotton gauze or pump spray once to twice daily. Debridement can be performed if lesions bleed or crust.
Systemic therapies are indicated for immunotherapy- or chemotherapy-responsive tumors. Treatment follows the regimen appropriate for the primary metastatic malignancy. Electrochemotherapy combines the administration of nonpermeable or poorly permeable highly intrinsic cytotoxic drugs with the application of short and intense electric pulses to the tumors to facilitate drug delivery into the cancer cells.
The patient deferred follow-up and passed away after 3 months of initial presentation. Cutaneous metastases are usually suggestive of disseminated illness and have a poor prognosis. Thus, early diagnosis and therapy are necessary. Similar limitations are the diagnosis of secondary infiltrates of presumed poorly differentiated carcinoma. Owing to a lack of immunohistochemistry and inability to perform a thyroid biopsy, we could not explicitly state that this was cutaneous metastasis from thyroid carcinoma, but the physical examination and other diagnostics supported the high likelihood of this diagnosis.