Study design
This is an observational prospective and longitudinal single-center study (cohort registration: https://www.chictr.org.cn/showproj.html?proj=194426..). In addition, to explore the potential hypothesis that high-dose FAS intake induces visceral fat accumulation and further affects GDM by regulating LXR-α/SREBP-1c pathway, we also conducted a 1:1 nested case-control study according to age ± 2 years old from the participants.
Finally, animal experiment was established to confirm the underlying mechanisms that high-dose FA leads to visceral fat accumulation and insulin resistance by regulating the LXR-α/SREBP-1c pathway, reveals the association between high-dose FA and the GDM risk.
The study was approved by the Ethics Committee of Qingdao Women and Children’s Hospital (Number: QFELL-KY-2022-15), all experiments were performed in accordance with ARRIVE guidelines. Each adverse event will be reported in writing to the Ethics Committee and each revision of the study protocol will also be reported. Investigators will strictly abide by the stated commandments of Helsinki and the ethical issues of human biomedical research when conducting this study. The patient has given informed consent to participate in the study, and patient information will remain confidential throughout the study process.
Recruitment of participants
Study recruitment started on 20 February 2023 at Qingdao Women and Children’s Hospital and it is expected to last until 1 May 2026. It will have to meet the following criteria:
Inclusion criteria
(1) Archived prenatal examination in the study hospital, and the first prenatal examination is 9–14 weeks gestation; (2) 18 years old and above; (3) Pregnant women living in Qingdao city;
Exclusion criteria
(1) Multiple pregnancy; (2) No clear time of last menstrual period; (3) Liver, kidney and other endocrine diseases; (4) Combined with heart, cerebrovascular diseases and other important organ dysfunction; (5) Those who are unable to complete follow-up as prescribed;
Data collectionResearch questionnaire form
Demographic covariates were obtained using a self-reported questionnaire, including age, education level, parity, personal monthly income and working status, personal and family disease history, smoking and drinking habits, daily physical activity. Dietary FAS intake (type, brand, duration, frequency and dose). Dietary folate data were estimated by a semiquantitative food frequency questionnaire (FFQ) [15].
Physical measurement
At the first prenatal visit during weeks 9 to 13+6 of pregnancy, body weight (to the nearest 0.1 kg), as well as height, waist circumference and hip circumference (to the nearest 0.01 cm) using a scale with a digital stadiometer were be measured by trained staff, Solo Digital Clinical Scale (Detecto, MO, USA). Blood pressure will also be measured by using an internationally certified Omron electronic sphygmomanometer (HEM-7220). VF depth will be scanned using a 5 − 2 or 9 MHz probe on a Philips iU22 ultrasound machine, is defined as the distance between the internal face of the external abdominal muscle and the anterior wall of the aorta [16]. Standardized measurement method has greatly reduced the potential data deviation, guarantee the actual reliability of the data.
Biochemical profile
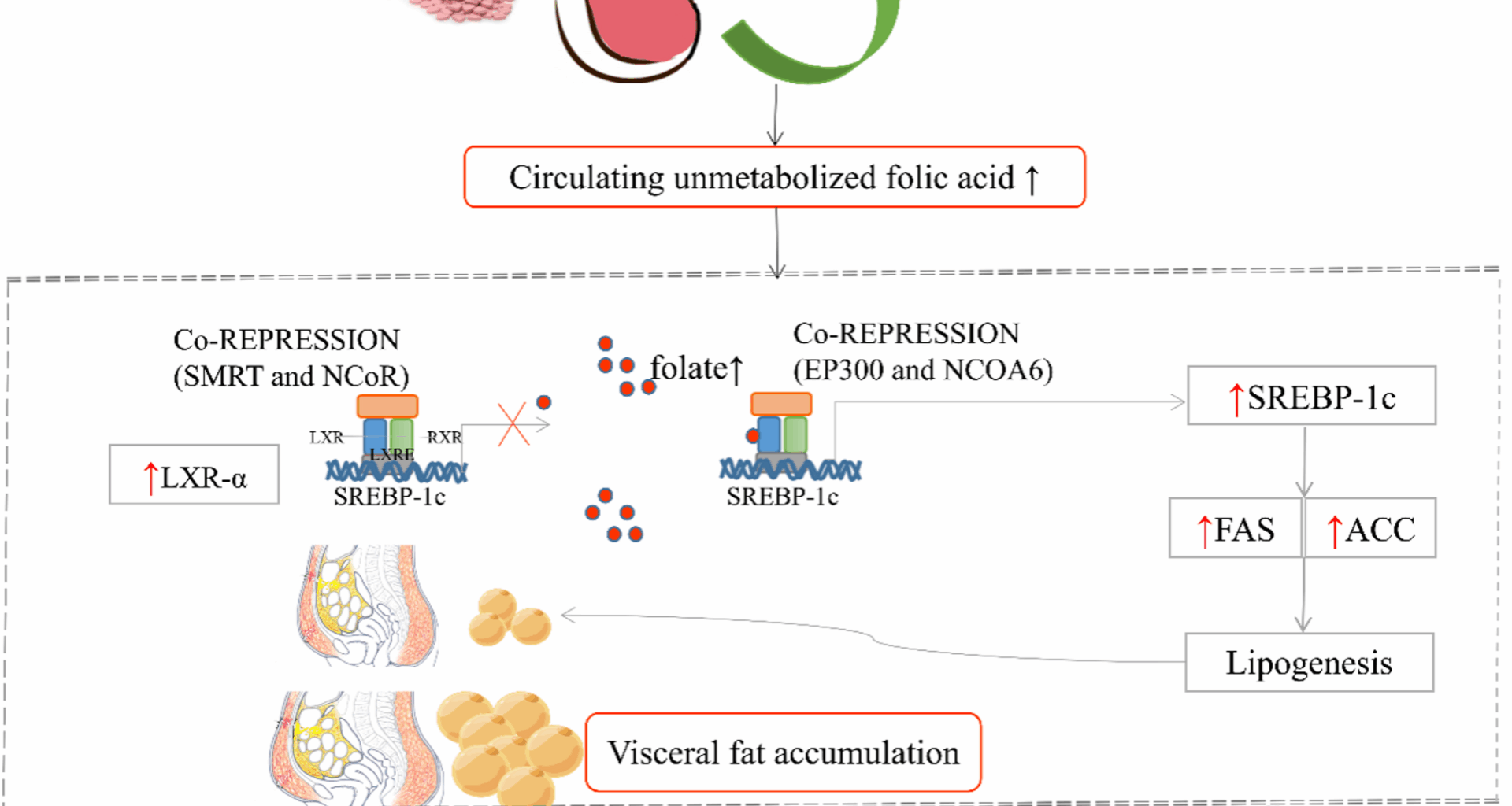
Before peripheral blood samples were collected, all subjects would be asked to fast overnight (at least 8 h) at the first prenatal visit (9–13+6 weeks of gestation). An accredited laboratory will analyze routine blood biochemical parameters such as RBC folate and serum vitamin B12 (VitB12) concentrations would be analyzed using chemiluminescence, as well as serum folate and methylmalonic acid (MMA) concentrations were determined by UPLC-MS/MS liquid chromatography tandem mass spectrometry. Based on the laboratory methods of enzyme-linked immunosorbent assay (ELISA) kit, the LXR-α/SREBP-1c levels will be sequenced between pregnant woman with GDM and without GDM.
Plan of follow-up
Pregnancy disease, smoking and drinking habits, daily physical activity. Dietary FAS intake (type, brand, duration, frequency and dose) and dietary folate data were collected again at 20 and 24–28 weeks of gestation. GDM were screened at 24–28 weeks’ gestation and we organized a 1:1 nested case-control study according to age ± 2 years old from pregnant women, the LXR-α/SREBP-1c levels between pregnant women with GDM and without GDM would be analyzed. The research schedule and procedures were presented in Fig. 2; Table 1.
Fig. 2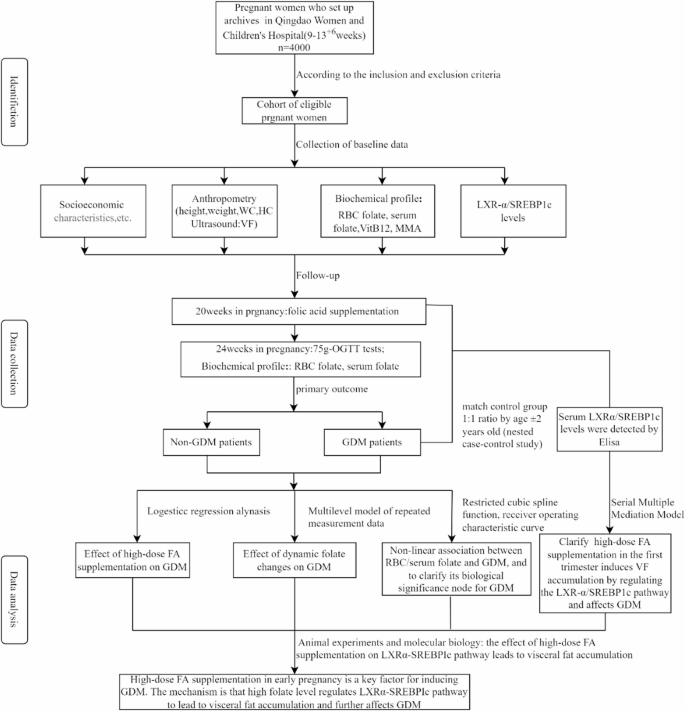 Table 1. Schedule of enrolment, follow-up and assessmentsOutcome measures
Table 1. Schedule of enrolment, follow-up and assessmentsOutcome measures
Participants were screened for GDM with the 75-g 2 h oral glucose tolerance test (OGTT) according to the International Association of Diabetes and Pregnancy Study Groups criteria[17]: Those with one of the elevated blood glucose values reaches the standard (i.e., fasting plasma glucos≥5.1mmol/L, 1-h plasma glucose≥ 10.0mmol/L, or 2-h plasma glucose≥8.5mmol/L.
Sample size calculation
In this study, high folate level was selected as the main exposure indictor to calculate the sample size. Based on previous study, we estimated that the incidence of GDM in first- trimester women with high RBC folate (≥600 ng/ml) was 22.0%[18]. sample size has been calculated to reach a confidence level of 95% with two-sided alpha = 0.05 and power=80%. According to the following formula:
\(N\;=\:\frac{\left(z_\alpha\sqrt{2\overline{pq}}+z_\beta\sqrt{p_0q_0+p_1q_1}\right)^2}{\left(p_1-p_1\right)^2}\)
The GDM group was p1 = 0.22, q1 = 0.78 and the control group was p0 = 0.1585, q0 = 0.8415, p¯ =0.189, q¯ =0.811. If we assume a dropout rate of 10%, taking into account the ineligible samples and scaling up sample proportion of cluster sampling to 1.7 times. In order to fully analyze the risk factors of GDM and lay the foundation for the follow-up cohort, 2000 people were finally determined in each group.
Based on the above information, a nested case-control study was designed to analyze the LXR-α/SREBP-1c levels between pregnant women with GDM and without GDM. Briefly, we will select all GDM patients among members of the cohort and match them with control group who reach the age ± 2 years at the time with ratio of 1:1.
Animal experimentsDiets and groups
Wild-type (LXR-α+/+) and LXR-α systemic knockout (LXR-α-/-) rats were constructed by CRISPR/Cas9 gene editing technology. Knockout rats were divided into 6 groups according to the FAS dosage: High-dose FAS group (HFA), Adaptive-dose FAS group (AFA), Low-dose FAS group (Con), LXR-α-/- rat high-dose FAS group (LXR-α-/–HFA), LXR-α-/- rat Adaptive-dose FAS group (LXR-α-/–AFA), LXR-α-/- rat low-dose FAS group (LXR-α-/–Con).
Based on the standard dietary feed AIN-93G, this project designed three kinds of FAS dosages (all purchased from Nanjing Telofei Feed Technology Co., LTD.): FA2 (LAD-3001G-F2) was FA 2 mg/kg (standard rodent feed); FA5 (LAD 3001G-F5) was FA 5 mg/kg; FA40 (LAD-3001G-F40) was FA 40 mg/kg feed. The dosage of FA2, FA5 and FA40 are equivalent to 400 µg, 800–1000 µg and 4 mg of FAS per day for pregnant women, respectively.
After the female rats feeding with different dosages of FA for 14 days, the female rats and the male rats were caged in a ratio of 2:1 for 72 h. The vaginal plug of the female rats was checked at 6:00 a.m. and 8:00 p.m. per day. The vaginal plug was recorded as gestation day 1 (GD1), and the pregnant female rats were included in this experiment. The female rats were weighed daily.
Detection
(1)
Serum folate, RBC folate, fasting blood glucose level (fasting blood glucose of pregnant rats was 5 h), and serum LXR-α and SREBP-1c protein expression levels were detected before feeding FA, 14 days after feeding FA, 14 days after GD14 and before killing pregnant rats (GD18).
(2)
OGTT: OGTT was performed on GD14, and blood glucose was measured after 5 h of fasting in pregnant rats. The dose of glucose was 1.2 g/kg* body weight. Blood glucose level of tail vein blood was measured by Abbott blood glucose meter at 30, 60, 120, and 180 min after glucose gavage.
(3)
Pregnant mice were anesthetized with chloral hydrate and killed on GD18. Visceral fat tissues, liver, pancreas and other tissues of pregnant mice were collected, mesenteric fat, retroperitoneal fat, inguinal fat, ovarian fat and other visceral fat were separated and weighed to observe the changes of visceral fat. A part of the collected tissues was fixed in formalin fixative for morphological examination. A portion of the collected tissues were quickly placed in liquid nitrogen and then transferred to a − 80 °C refrigerator for use in molecular biology assays.
(4)
HE staining was used to detect the changes of adipocytes in liver and visceral adipose tissues.
(5)
Enzyme-linked immunosorbent adsorption test (ELISA) was used to detect fasting serum insulin levels in pregnant rats.
(6)
RT-PCR and Western blot were used to detect the expression of LXR-α, SREBP1c, fatty acid synthase and acetyl-coa carboxylase in liver and visceral adipose tissues.
(7)
Immunohistochemistry was used to detect the localization of LXR-α, SREBP-1c, fatty acid synthase and acetyl-coa carboxylase in liver and visceral adipose tissues.
Statistical analysis
Baseline RBC folate levels were divided into high-dose FA group (≥ 600 ng/ml), moderate-dose FA group (400 ~ 600 ng/ml) and low-dose FA group (< 400 ng/ml), serum folate levels were grouped the quartile. Binary logistics regression model was used to investigate the effect of high-dose FAS on GDM risk. Multilevel model of repeated measurement data was used to determine the association between the dynamic folate levels changes and GDM risk.
The log-likelihood ratio (LL), the Akaike Information criterion (AIC), the correctional Akaike information criterion (AICc) and the Bayesian information criterion (BIC) were combined to select the optimal model, restricted cubic spline (RCS) and the optimal model analysis were utilized to determine the biological significance node of RBC/serum folate for GDM. Receiver operator characteristic (ROC) curve analysis and logistic regression model were performed to evaluate the ability of serum folate and RBC folate levels to predict GDM. Delong tests were used to compare the area of receiver operator characteristic (AUC).
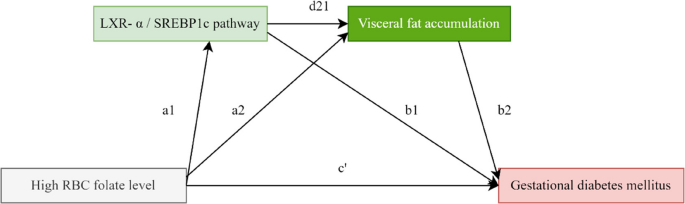
Serial multiple mediation model was constructed using the mediation formula method to clarify how high-dose FAS in early pregnancy causes VF accumulation and further affects GDM by regulating the LXR-α/SREBP-1c pathway. (Fig. 3). Statistical analyses are analyzed using SAS software with statistical significance set at 2-sided P < 0.05.
Serial multiple mediation model of high RBC folate level on GDM risk
RBC folate level is an important indicator of long-term nutritional status of folic acid. It could reflect pre-pregnancy folate level because it is collected in early pregnancy. Serum folate reflected the level of early pregnancy. High RBC (pre-pregnancy)/Serum folate (early pregnancy) level – LXR-α/SREBP1C pathway – Visceral fat accumulation – GDM (24–28 weeks of gestation). Chain indirect effects = a1d21b2; The total indirect effects = a1d21b2 + a2b2 + a1b1; The direct effect = c’; The total effects (direct + indirect) = a1d21b2 + a2b2 + a1b1 + c’.
Study status
Recruitment has launched in 20 February 2023 and it is estimated to take 2 years for full recruitment.