Study design and sample collection
Based on geographical distribution, Shandong Province was divided into three distinct regions, eastern (Qingdao, Yantai, Weifang, Weihai, and Rizhao), central (Jinan, Zibo, Dongying, Tai’an, and Linyi City) and western (Zaozhuang, Jining, Dezhou, Liaocheng, Binzhou, and Heze City). Annually, one or two cities from each region were randomly selected using the method of computer-generated random numbers, and within these cities, the same random selection process was applied to identify one county (district) as the survey sites. The determination of the required sample size for this study was based on the formula n = Z2P(1-P)/ε2. P represented 50% of pertussis seropositivity with a relative permissible error (ε) of 3% and a confidence interval of 95%. When the ratio of lost follow-up of 10% was taken into account, a total of 1174 participants were needed to be enrolled per year. Participants enrolled in the study were healthy individuals who didn’t exhibit symptoms of respiratory infection, had not used antibiotics in the three months preceding the survey, and had been residing at the survey site for at least 12 months. Participants with a primary or secondary immunodeficiency disease and those who had received blood, blood components, or immunosuppressive agents within the 6 months prior to enrollment were excluded. The participants were stratified into six age-based subgroups (< 3, 3 ~ 5, 6 ~ 12, 13 ~ 16, 17 ~ 19, and ≥ 20 years). Within each age group in one site, 30 to 50 individuals were random selected, ensuring a balanced gender distribution in a ratio of 0.9 ~ 1.1. Serum specimens were collected from each participant, and demography and epidemiology information, including name, gender, age, residential area, medical history, and vaccination status of pertussis, were meticulously recorded in the questionnaire (Zhang Y: Questionnaire of seroepidemiological survey, unpublished) specifically designed for this study. To guarantee the precision and uniformity of data collection, the questionnaire was crafted through relevant literature reviews, interviews among study population, and multiple rounds of content validity evaluations by experts. Prior to conducting the survey, interviewers were properly selected, briefed, and trained, and a small sample of our target population was selected to pilot and evaluate. A total of 9,238 datasets were collected from 2017 to 2023.
Laboratory testing
Specimens were assayed for PT-IgG utilizing the enzyme-linked immunosorbent assay (ELISA) method at the laboratory of Shandong Provincial Center for Disease Control and Prevention. A commercial kit (EUROIMMUM Medizinische Labordiagnostika AG, Germany) was used, with strict adherence to the manufacturer’s protocols. Antibody activities were quantified in International Units/mL (IU/mL) and referred to the WHO international standard for human pertussis antiserum (NIBSC 06/140). Currently, there are no established cut-off values for PT-IgG that serve as a definitive indication of Bordetella pertussis infection in China. For this study, through integrating the guidelines provided by the ELISA kit manufacturer for interpreting results, along with findings from previous research [10, 11], we defined PT-IgG concentration of ≥ 40 IU/mL as seropositivity, suggesting a recent pertussis infection within the past year in the absence of pertussis vaccination, and PT-IgG concentration of ≥ 100 IU/mL was considered diagnostic evidence of an acute or recent infection if the individual had not received a booster dose of the pertussis vaccine within the preceding year. PT-IgG concentration < 40 IU/mL indicated no evidence for a previous infection of Bordetella pertussis.
Reported incidence of pertussis
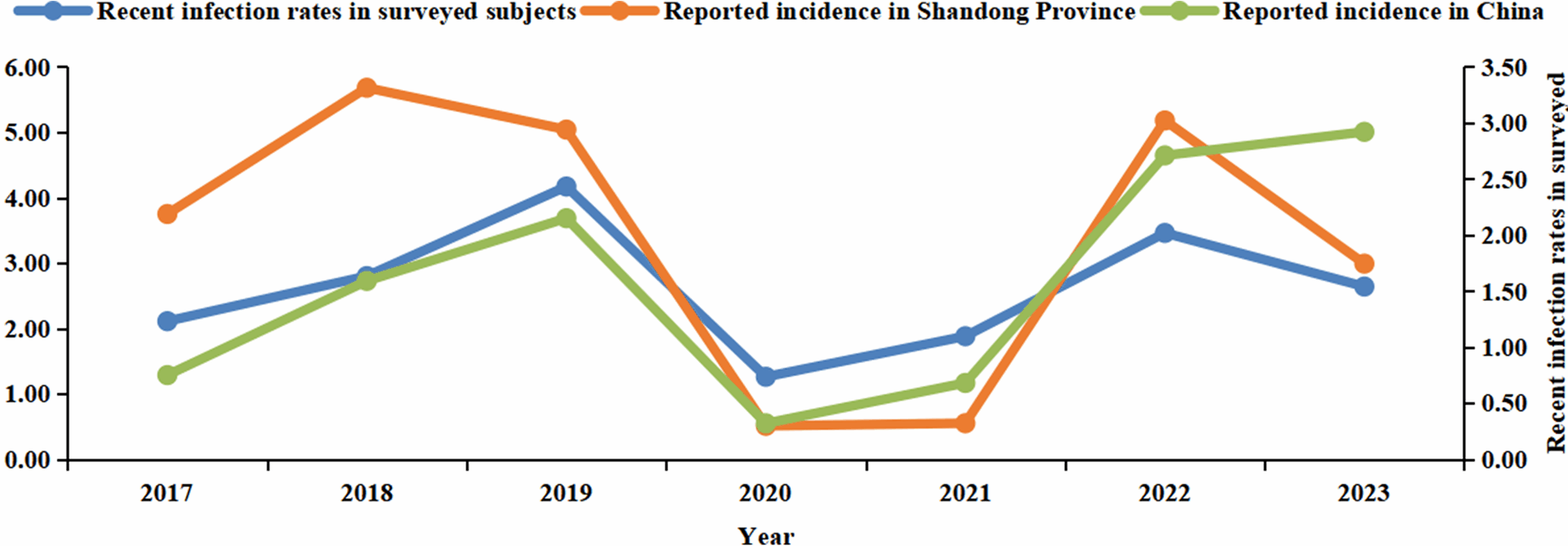
Data on pertussis cases were obtained from the National Notifiable Disease Reporting System (NNDRS). To calculate the incidence rate of pertussis, we divided the number of reported cases by the total population, with the rate expressed per 100,000 individuals. The population figures used as the denominator in these calculations were sourced from the Shandong Statistical Yearbook [12]. From 2017 to 2023, the population of Shandong Province maintained a relatively stable level, fluctuating between 101 million and 102 million.
Estimated incidence of pertussis
The incidence rate of pertussis infection was estimated using the method described in established literature [11]. It was observed that following infection, the PT-IgG concentration typically takes 58.6 days to drop to a level of 100 IU/mL and 365 days (12 months) to reach a level of 30 IU/mL. With the threshold of 100 IU/mL, the incidence of infection was calculated using the formula: 365.2/58.6 × the proportion of subjects with PT-IgG concentration at or above 100 IU/mL. To ensure the accuracy of incidence assessment, it was essential to consider the potential interference of recent vaccination with pertussis-containing vaccines. Therefore, the incidence estimation excluded individuals who had received such vaccinations within the past 12 months. Taking into account the current immunization schedule in China, which includes 4 doses of pertussis vaccine administered at 3, 4, 5, and 18–24 months of age, the incidence estimation of pertussis infection in this study was confined to individuals aged three years and above.
Statistical analysis
Data analysis was conducted utilizing Microsoft Excel 2020 and IBM SPSS Statistics 26 software. Quantitative data were summarized using means and their associated 95% Confidence Intervals (CI), and qualitative data were presented as percentages. The chi-squared test was used to assess variations in rates or composition ratios among different time periods, regions, age groups, and genders within the population. A significant level of p < 0.05 was used to determined statistical significance. The Spearman correlation coefficient r was utilized to evaluate the linear correlation relationship between the positive rate of PT-IgG antibodies and the reported pertussis incidence across various age groups and regions.