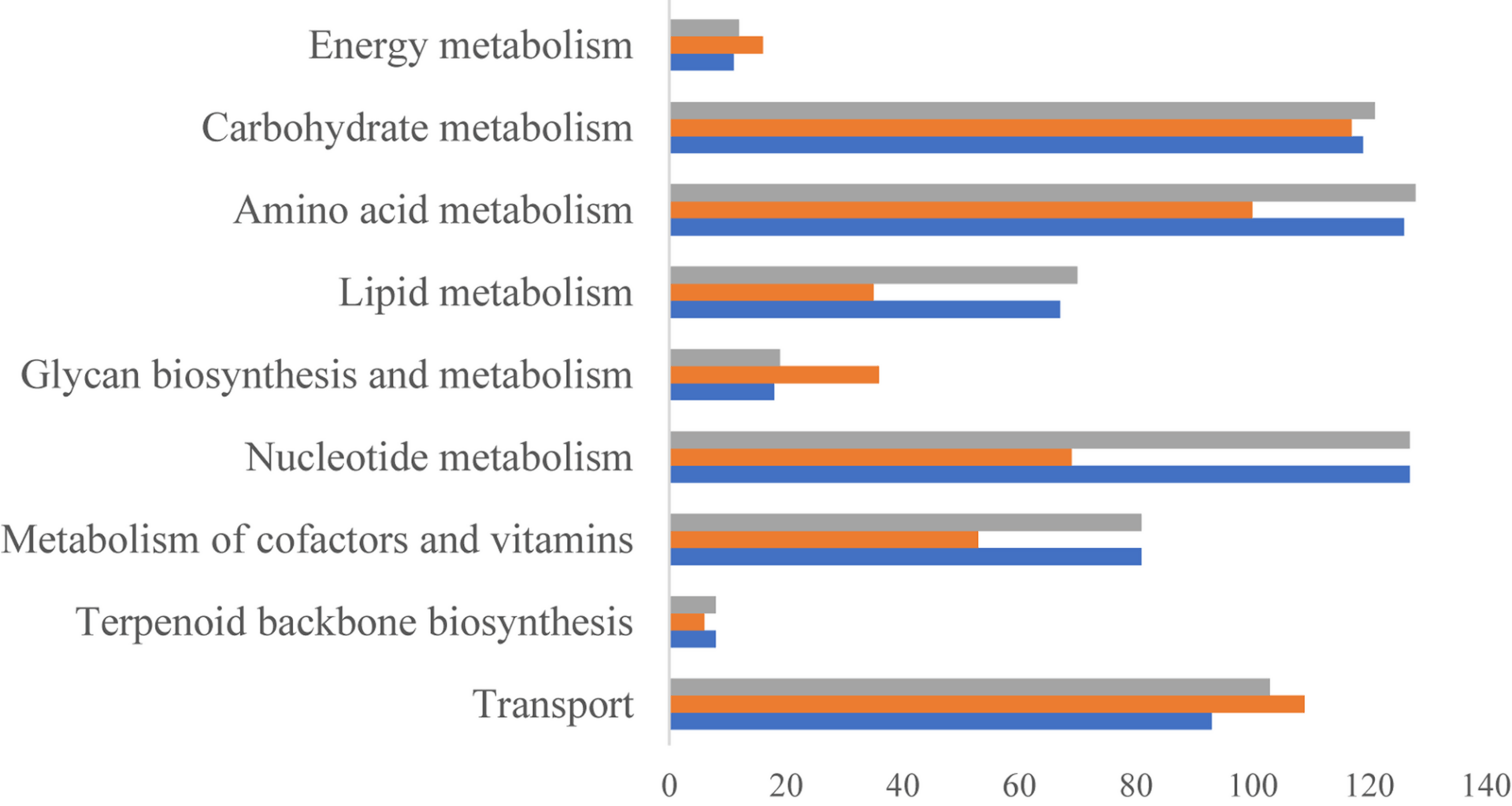The reconstruction and characteristics of the genome-scale metabolic model for Streptococcus suis
Streptococcus suis SC19 strain is a hypervirulent serotype 2 isolate that causes disease in pigs and humans. Due to its pathogenicity, SC19 represents a potential candidate for vaccine development [29]. Gene-protein reactions of S. suis were mainly based on the automated ModelSEED pipeline and homologous comparison with template GSMMs. The SEED model started with the RAST annotation of the S. suis genome, which contained 392 genes, 988 metabolites, and 822 reactions. There were 269, 238, and 335 homologous genes in S. suis with the B. subtilis metabolic modelv3 [30], the Staphylococcus aureus model iYS854 [31], and the Streptococcus pyogenes model [12], respectively. Four hundred and seventeen genes, 692 reactions, and 673 metabolites were then extracted from the three template models. After integration with the SEED model and unification of the format of the metabolites and reactions, the draft model of S. suis included 477 genes, 714 metabolites, and 816 reactions. The draft model contained cytoplasmic and extracellular compartments, which were the same as those of all the template GSMMs. Of the 238 transporters of S. suis annotated using the TCDB, 98 were included in the draft models. Thirteen transporters were newly annotated, rather than using template models. To enable the model to simulate growth, 92 metabolic gaps in the biosynthesis of biomass precursors were manually filled according to the literature or Kyoto Encyclopedia of Genes and Genomes pathways. In the model iNX525, there were 31 orphaned reactions and 73 dead-end gaps. Since the presence of these gaps does not affect biomass biosynthesis, they do not require filling in the current model. Excluding 22 exchange reactions and one demand reaction, the remaining blocked reactions exhibited a high gene coverage rate (98.23%), indicating that the current model is fully annotated. The final genome-scale metabolic model for S. suis, iNX525, contained 525 genes, 708 metabolites, and 818 reactions (Supplementary file 2 and Table S2). These reactions were distributed across 61 metabolic pathways. The main 10 metabolic subsystems are shown in Fig. 1. The ratio of gene-associated reactions in the 10 metabolic subsystems averaged 96.9%, which reflected adequate annotation of the genome using the model iNX525. Gene coverage for central carbon metabolism, metabolism of cofactors and vitamins, metabolism of other amino acids, nucleotide metabolism, and terpenoid backbone biosynthesis reached 100%. Gene coverages for carbohydrate metabolism and amino acid metabolism were more than 98%. The top three reactions were amino acid, nucleotide, and carbohydrate metabolism. The top three genes were involved in carbohydrate transport and amino acid metabolism. The number of reactions and genes involved in carbohydrate and amino acid metabolism was relatively large, which was reflected in the diversity of the two metabolic subsystems. In carbohydrate metabolism, the metabolic pathways with more than 20 reactions were starch, sucrose, and galactose metabolism. Galactose assimilation and metabolism influence capsular formation and the cariogenicity of Streptococcus [32, 33]. Starch and sucrose influence intracellular polysaccharides and biofilm development in Streptococcus [34]. Cysteine and methionine metabolism had the most reactions in the amino acid metabolic subsystem. Metabolic subsystems with more genes than reactions included glycan biosynthesis and metabolism, energy metabolism, and transport subsystems. S. suis lacks several reactions in its respiratory chain, which compels it to inhabit nutrient-rich niches. The 109 transporters annotated for 95 metabolites suggested a plentiful transport system in S. suis for nutrient absorption. Glycan biosynthesis includes peptidoglycan and o-antigen nucleotide sugar biosynthesis, which provides capsular polysaccharide components (such as CMP-N-acetylneuraminate and UDP-alpha-D-galactofuranose) in S. suis [35].
Genes, reactions, and gene-associated reactions for each metabolic subsystem of the model iNX525
The model iNX525 was compared to two other Streptococcus GSMMs. The levels of coverage of the annotated open reading frames in the three models were 28.7% (iNX525), 28.2% (S. pyogenes’ model), and 25.1% (iSMU). A total of 560 metabolites in model iNX525 were also present in either of the two Streptococcus models (Table S3), and they took part in 646 reactions (excluding exchange reactions). In addition to Streptococcus’ primary metabolism, the shared metabolic reactions included three reactions in H2O2 metabolism, and four reactions in O-antigen nucleotide sugar biosynthesis. The reactive oxygen species (ROS) produced by immune cells are highly reactive and indiscriminately damaging in response to pathogens [36]. Streptococcus can reduce superoxide to H2O via H2O2 metabolism, which helps to detoxify ROS and adapt to hosts under conditions of oxidative stress [37]. The anabolism of dTDP-L-rhamnose from D-glucose-1-phosphate was found to be broadly distributed in Streptococcus, and this might be related to the biosynthesis of the serotype-specific polysaccharide antigen [38]. Compared to the S. pyogenes and S. mutans models, model iNX525 contained 54 unique metabolites (Table S4). The largest number of unique metabolites was distributed in amino acid biosynthesis, reflecting bacterial differences in streptococcal amino acid metabolism [39]. The 14 unique metabolites in amino acid metabolism included four in cysteine and methionine metabolism; three in glycine, serine, and threonine metabolism; three in lysine biosynthesis; two in valine, leucine, and isoleucine biosynthesis; and L-arogenate and N-acetylputrescine. Six unique amino sugar and nucleotide sugar metabolites were involved in chitobiose hydrolysis and sialic acid biosynthesis. Ten unique metabolites of pentose and glucuronate metabolism were mainly associated with D-glucuronate conversion. Ten unique metabolites may be related to the de novo synthesis of certain cofactors, mainly including five in thiamine metabolism, two in riboflavin metabolism, glutaryl-[acp] methyl ester, and so on. Nine unique metabolites of nucleotide metabolism were all related to 2’,3’-cyclic-nucleotide phosphodiesterase/nucleotidase. 2′,3′-cNMP levels affect cell growth, biofilm formation, and other bacterial phenotypes. Proteins with phosphodiesterase activity against cyclic di-AMP affect the virulence of S. suis [40].
Essential gene analysis
For S. suis, the Database of Essential Genes (DEG) lists 361 conditionally essential genes required for the infection of pigs [41]. One hundred and fifty-nine genes were identified as essential for growth in brain-heart infusion (BHI) medium using transposon mutagenesis [42]. Model iNX525 identified 122 essential genes (Table S5) with 71.6% and 76.3% accuracy, respectively, matching the two published mutagenesis datasets. As shown in Fig. 2, iNX525 performed better at predicting essential genes than non-essential genes, as indicated by its high precision (81%) and sensitivity (82% and 79%, respectively), but comparatively low specificity (39% and 49%, respectively). Overall, the finding verified the prediction capacity of iNX525. The discrepancy between iNX525 and published data might be related to the uncertainty in nutrient absorption in complex habitats and the lack of regulatory constraints on the metabolic model. Additionally, non-metabolic genes involved in processes such as DNA replication, RNA transcription, and protein synthesis were not included in iNX525, but were essential in the experimental data.
iNX525 essentiality predictions align with two experimental studies
In addition to assessing model quality, gene essentiality analysis aids in identification of potential antimicrobial drug targets. Sixty-two genes predicted by iNX525 were identified as essential from the experiments [41, 42]. They were involved in 80 reactions (Table S6) distributed across the following seven metabolic subsystems: lipid metabolism (21 reactions), carbohydrate metabolism (14), transport (13), amino acid metabolism (10), glycan biosynthesis and metabolism (9), metabolism of cofactors and vitamins (8), and nucleotide metabolism (5). Specifically, SSU1607, SSU1602, and SSU1605 are required for fatty acid biosynthesis. Several acyltransferases involved in glycerolipid or glycerophospholipid metabolism are essential and are characterized as drug targets in S. suis [43]. Glycolysis and the pentose phosphate pathway (PPP) are pivotal for central carbon metabolism in S. suis [44]. The formation of L-lactate and malonyl-CoA is essential in pyruvate metabolism. The model iNX525 had no solution space after removing exchange reaction of L-lactate, which represented the metabolic characteristics of lactic acid bacteria. Malonyl-CoA is required for fatty acid biosynthesis. Glucosamine-6-phosphate synthase (SSU0500) and UDP-glucose 4-epimerase (SSU1258) are essential for amino and nucleotide sugar metabolism. Carbonic anhydrase (SSU1820) is essential for S. suis growth in ambient air [45]. Essential transport genes are involved in the transport of phosphates (SSU0948–SSU0953), metals (SSU1865–SSU1869), amino acids (SSU0502, SSU1852, and SSU1853), and vitamins (SSU0292, SSU1506, and SSU1764). SSU0319, which encodes alanine transaminase and L-aspartate 4-semialdehyde conversion from threonine and L-aspartate, is essential for amino acid metabolism. Although S. suis is auxotrophic for several amino acids and requires exogenous amino acids, any type of amino acid can be synthesized using other nutrients in the CDM. Furthermore, peptidoglycan synthesis requires D-amino acid metabolism. The biosynthesis of polyketide sugar units and peptidoglycans is essential for S. suis growth and contributes to the composition of the capsule, cell wall, and membrane. Biotin carboxyl carrier protein formation, nicotinate phosphoribosyltransferase in the conversion of nicotinate to nicotinamide, FolD bifunctional protein in the folate cycle, and dephospho-CoA kinase for CoA production are essential in the metabolism of cofactors and vitamins. Guanylate kinase (SSU0378), uridylate kinase (SSU1160), and thymidylate synthase (SSU0778) are essential in nucleotide metabolism.
Nutrition utilization using the model
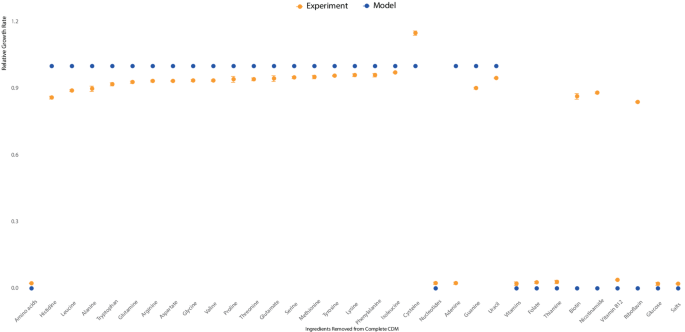
S. suis is a common colonizer of the upper porcine respiratory tract and can cross the blood–brain barrier to reach the brain and cause meningitis [46]. S. suis adapts and survives in various nutrient niches. Cell growth phenotypes are generally used to evaluate the predictive ability of genome-scale metabolic models [47]. Growth experiments of S. suis were performed on CDM with glucose as the carbon source [48]. Carbon source utilization was predicted by changing a single carbon source in the CDM via exchange constraints to the model and optimizing biomass production using the flux balance analysis (FBA). Simulations using iNX525 predicted no growth in CDM lacking glucose. Twenty carbohydrates were individually added to the CDM to determine whether S. suis could use these carbon sources for growth. S. suis was able to grow on glucose, fructose, mannose, maltose, lactose, trehalose, sucrose, N-acetylglucosamine, starch, glycogen, D-raffinose, and melibiose, but not on mannitol, xylitol, xylose, α-methylglucoside, D-ribose, L-arabinose, sorbitol, or inulin. The simulation results exactly matched those reported in the literature [48].
The effects of genetic perturbations on cell growth were qualitatively evaluated using iNX525. For the 12 available carbon sources in the CDM, the in silico growth phenotypes of msmk knockouts were in accordance with those of the 11 types of carbon sources reported in the literature [48]. The deletion of msmk led to no cell growth on D-raffinose or melibiose because the utilization of the two sugars relied on the ABC transport system (SSU1372, SSU1371, SSU1370, and SSU1701). Cell growth on nine types of carbon sources was not affected after deleting msmk because the nine carbon sources could be utilized by other transport systems, such as phosphotransferase systems or extracellular hydrolysis. In the CDM, using glycogen as the carbon source, the in silico cell growth rates decreased by 79%, whereas the in vivo cell growth was completely abolished. Glycogen is converted to maltotetraose and maltose by amylopullulanase in iNX525. If the product of glycogen degradation was maltotetraose, in silico msmk knockout would lead to no growth in the CDM using glycogen as a carbon source. GlnA, encoding glutamine synthetase, is required for full virulence in S. suis [49]. After deleting glnA, the in silico cell growth rates were not affected under unconstrained glutamine uptake, but decreased from 0.242 h−1 to 0.16 h−1 by constraining the glutamine uptake rates (0.1 mmol/g DW/h). The change in growth rate was completely consistent with bacterial growth with or without 0.2% glutamine in TSB medium [49].
CDM containing glucose is usually used as the minimal medium for S. suis. CDM also contains amino acids, vitamins, nucleotides, inorganic salts, carbon sources, and phosphorus sources. To explore autotrophies in S. suis, the in silico growth phenotypes were simulated by removing components from the CDM using the model iNX525. As shown in Fig. 3, the elimination of 19 amino acids from the CDM abolished S. suis cell growth, but the removal of individual amino acids did not. The S. suis genome encodes metabolic pathways involved in amino acid interconversion. Cys deficiency increases the growth rate by almost 15% in S. suis. Cysteine production may result from serine conversion, with methionine or another sulfate as the sulfur source. In a superior, less stressful experimental setting, S. suis might not need to produce GSH to resist stress via cysteine catabolism, which could free up more energy and carbon sources for cell growth. S. suis can theoretically synthesize nucleotides using both oxidative and non-oxidative branches of the PPP. The model predictions showed that adenine removal did not affect cell growth, but that exogenous adenine was required for growth in vivo. This disagreement might be because several genes related to the synthesis of ribose-5-phosphate in the PPP are downregulated or not expressed in minimal medium [50]. All six vitamins were predicted to not be synthesized de novo by iNX525. The lack of folate, thiamine, or vitamin B12 abolished in vivo growth. Removing riboflavin, biotin, or niacinamide inhibited in vivo growth, which could be related to the differing quantities of vitamins required by S. suis. When precursors or other active forms of vitamins are added, the corresponding vitamin components in the biomass equation can be synthesized. For example, riboflavin and niacinamide were transformed into FAD and FMN, and NADPH and NADH, respectively. An investigation of nutritional requirements using the model iNX525 deepened our understanding of the growth and metabolism of S. suis and will facilitate the design of more refined minimal culture media for S. suis.
Model predictions (blue) match growth experiments for S. suis in defined medium (yellow)
Metabolism-related virulence factors (VF) analysis
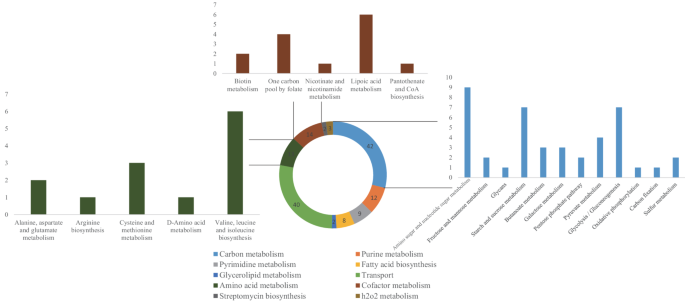
In model iNX525, a total of 131 genes deduced from the virulence-linked gene database (Table S7) based on sequence similarity were mapped to model iNX525, and 79 genes were linked to 167 metabolic reactions (Table S8). As shown in Fig. 4, these were mainly distributed in carbon metabolism (25%), transport (24%), nucleotide metabolism (23%), amino acid metabolism (11%), cofactor metabolism (9%), and lipid metabolism (6%). VFs in carbon metabolism covered 10 metabolic pathways, with the top three pathways being the metabolism of amino sugar and nucleotide sugar, glycolysis, and starch and sucrose. Transport metabolism included 28 ATP-binding cassettes and 11 phosphotransferase transport reactions. Phosphates, amino acids, biogenic amines, saccharides, and divalent metal ions are all imported by the ABC transporters. PTS transporters participate in sugar transport systems. Some transporters have emerged as VFs in bacterial cells [51, 52]. Ten VFs were involved in 37 nucleotide metabolic reactions. The five amino acid metabolic pathways identified were valine, leucine, and isoleucine biosynthesis (eight reactions); cysteine and methionine metabolism (five reactions); alanine, aspartate, and glutamate metabolism (SSU0738, SSU0737, and SSU0157); arginine biosynthesis (SSU0582); and D-amino acid metabolism (SSU0596). The five cofactor metabolic pathways included lipoic acid metabolism (six reactions), folate metabolism (four reactions), biotin metabolism (SSU1603), nicotinate and nicotinamide metabolism (SSU0860), and pantothenate and CoA biosynthesis (SSU1680).
The distribution of virulence-linked genes in the model iNX525
Apart from sequence similarities, genome-scale metabolic modeling on BHI medium in silico was used to identify VFs. The genes affecting each virulence-linked metabolites were identified by evaluating whether a given gene was essential for metabolite production. The virulence of S. suis is linked to suilysin, capsules, biofilms, and quorum sensing signaling molecules. Nine confirmed small molecules were selected for VF formation. Genes affecting VF synthesis overlapped and were independent, reflecting their differences in VF formation. Twenty-eight overlapping genes for all nine VFs (Table S9) were present at a high density in the central carbon metabolism pathway, including nine genes involved in both glycolysis and oxidative phosphorylation, four ABC and two PTS transporters, two genes involved in starch and sucrose metabolism, and two genes in glycan metabolism.
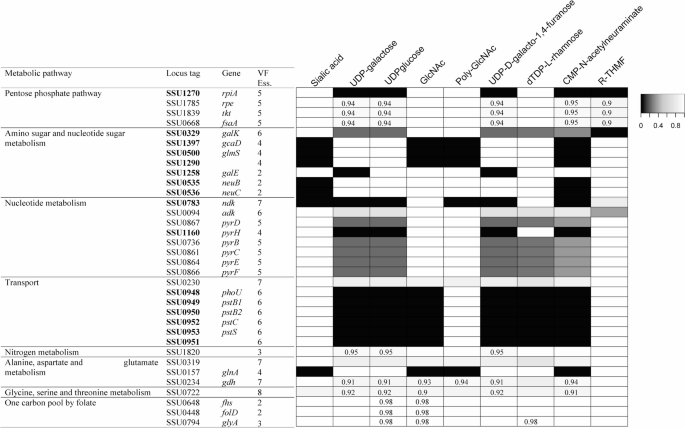
Thirty-four genes affecting the synthesis of at least two types of VFs were spread throughout a broad range of pathways (Fig. 5). Specifically, the production of D-ribulose 5-phosphate and ribose 5-phosphate from D-fructose 6-phosphate and glyceraldehyde 3-phosphate, and the interconversion of D-ribulose 5-phosphate and ribose 5-phosphate were in the PPP. In amino sugar and nucleotide sugar metabolism, four genes were involved in UDP-N-acetylglucosamine production from fructose 6-phosphate. In nucleotide metabolism, UTP/UMP synthesis from carbamoyl phosphate, nucleoside diphosphate kinase, nucleoside diphosphate kinase, and adenylate kinase have been shown to affect VF production. Phosphate transport was necessary for VF production. In the amino acid metabolism, enzymes related to alanine, aspartate, glutamate, glycine, threonine, and carbonic anhydrase were crucial for the virulence of S. suis. Folate metabolism was linked to the formation of UDP-glucose, N-acetyl-D-glucosamine, and dTDP-l-rhamnose. Although folate metabolism is predicted to have a smaller effect than the other metabolic pathways, its impacts on virulence have been confirmed in other pathogenic microorganisms [53].
Genes affected at least two kinds of virulence factor synthesis. Impact of a given gene’s deletion is shown as black indicating totally inhibition and white indicating no-effects
Impact of a given gene’s deletion is shown as black indicating totally inhibition and white indicating no-effects.
Among genes unique to one type of VF (Table S9), SSU0307, encoding poly-beta-1,6-N-acetyl-D-glucosamine synthase; SSU0563, encoding UDP-galactopyranose mutase; SSU0537 and SSU0538, encoding N-acylneuraminate cytidylyltransferase, were essential for the production of poly-N-acetyl-glucosamine, UDP-D-galacto-1,4-furanose, and CMP-N-acetylneuraminate, respectively. The seven genes essential for dTDP-L-rhamnose production comprised direct synthesis from D-glucose 1-phosphate during O-antigen nucleotide sugar biosynthesis, dihydrofolate reductase and thymidylate synthase in folate metabolism, and the transformation of dUDP and dTDP in pyrimidine metabolism. The most distinct genes were associated with R-THMF production, including three genes required for (4 S)−4,5-dihydroxy-2,3-pentanedione biosynthesis from l-methionine, four genes involved in converting l-aspartate into fumarate, and three genes involved in the conversion of L-citrulline to L-arginine.
Relationship between growth and virulence
To investigate the association between virulence and growth, virulence-linked genes from a virulence-linked gene database were compared with essential genes. S. suis survival in BHI medium, blood, brain, and porcine cerebrospinal fluid (CSF) required 158, 292, 243, and 152 genes, respectively, as determined using transposon mutants. The number of virulence-linked essential genes ranged from 47 in BHI medium to 102 in pig blood. The ratios of VF-linked essential genes were 29.7%, 34.9%, 32.5%, and 37.5% in BHI medium, blood, brain, and CSF, respectively. The number of essential genes was similar in BHI medium and CSF; however, the ratio of VF-linked essential genes was most divergent. This ratio was lower in BHI medium than in the other three conditions, possibly due to a lack of host selection pressure in BHI medium. Transposon insertion sequencing (Tn-seq) screens performed in infection models have shown that mutants that are unable to synthesize certain VFs are unable to colonize the infection site, implying that virulence-linked genes may be essential in some contexts but not in others (such as those in liquid culture).
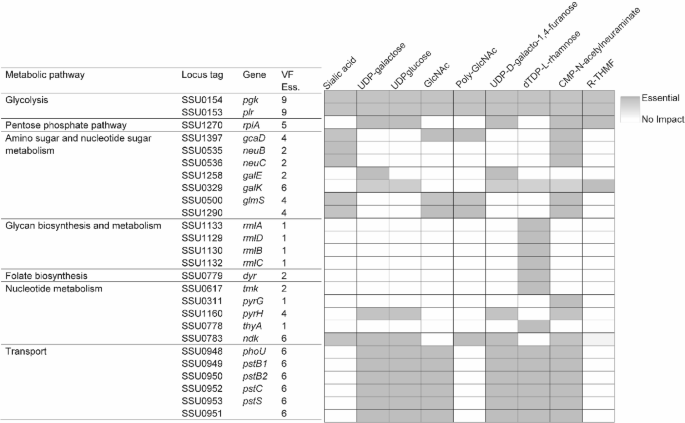
To analyze the potential overlap of virulence-linked genes with growth activity, in silico gene knockouts were used to assess the levels of growth inhibition and VF synthesis inhibition by normalizing the resulting biomass flux and VF flux to wild-type production levels. The 122 genes predicted by the model iNX525 as essential for growth on BHI medium were compared to the 34 genes essential for VF formation (Table S10), and 26 genes were discovered to be essential for both cell growth and the production of at least one VF. As shown in Fig. 6, amino sugar and nucleotide sugar metabolism had the most related genes, which covered the production of the listed VFs. SSU0153 and SSU0154, which are involved in glycolysis, were essential for all nine VFs, and SSU1270, which is involved in the PPP, was essential for five of the VFs, reflecting the robustness of glycolysis in central carbon metabolism [43]. During glycan biosynthesis and metabolism, the Rml operon genes are required for dTDP-L-rhamnose synthesis as components of the capsule and biofilm. Some genes involved in nucleotide metabolism hindered the formation of only one type of VF, while others inhibited the production of more types of VF. For example, SSU0783, encoding nucleoside diphosphate kinase was predicted to be required for six VFs [54]. In this transport system, the phosphate ABC transporter was predicted to be essential for six VFs. PstB has been found to provide protection against S. suis in mouse and pig models [55]. Ultimately, this analysis provides some genes ranked by their impact on virulence pathways, in addition to growth inhibition, which may aid in the design development of medicines with a broad impact on metabolic processes.
Genes essential for both cell growth and the VF production. Essentiality of a given gene’s deletion is represented by color intensity, with darker colors indicating stronger essentiality and white indicating non-essentiality