Key Insights
Disrupting the interaction of two cancer-driving proteins could help fight the disease.
Experiments with genetically engineered mice suggest that breaking up the protein partnership could be an effective strategy.
The new molecular breakers also block cancers driven by an unrelated protein, which is something of a mystery.
In the world of proteins that drive cancer, Ras proteins and PI3Kα (formally known as phosphoinositide 3-kinase alpha) are major players. Both are involved in processes that signal cancer cells to grow and proliferate. Although scientists have come up with drugs that can block one protein or the other, these have problems: cancers develop resistance to Ras inhibitors, and PI3Kα inhibitors can lead to blood sugar dysregulation. Now several companies are developing molecules that break up this protein power couple with the hopes of adding a new weapon to the cancer-fighting arsenal.
This year, BridgeBio Oncology Therapeutics (BBOT), Frontier Medicines, and Vividion Therapeutics have all disclosed they are developing small-molecule breakers for the Ras-PI3Kα interaction. Ras and PI3Kα are like a company’s CEO and chief financial officer, says BBOT’s chief scientific officer Pedro J. Beltran. “If they cannot talk to each other, everything just falls apart.”
“If you think of the glue as an open hand receiving Ras, we essentially turn that open hand into a fist that bounces Ras out.”
Pedro J. Beltran, chief scientific officer, BridgeBio Oncology Therapeutics
Frank McCormick, who studies Ras signaling at the University of California, San Francisco, says the interaction between Ras and PI3Kα has been known for many years, “but the significance in cancer has always been rather difficult to figure out.”
Julian Downward and colleagues discovered a key insight into this interaction back in 2007 when they genetically engineered mice so that their PI3Kα could no longer interact with Ras. Those mice were resistant to cancers caused by mutant Ras oncogenes (Cell, DOI: 10.1016/j.cell.2007.03.051).
“The idea was that if you could actually stop that interaction happening, that might be a good treatment for cancers caused by these mutant Ras cancer genes,” says Downward, who is now associate research director at the Francis Crick Institute.
What’s more, McCormick, who was not involved in that research, points out that the genetically engineered mice were quite healthy. Downward had “established using genetics that interrupting that interaction could have therapeutic value and would be safe, at least in mice,” McCormick says.
The problem is that Ras and PI3Kα interact weakly, which made it tricky to find a drug that could prevent the interaction, McCormick says. But as Downward and McCormick were thinking about preventing this interaction for cancer, scientists at Daiichi Sankyo were trying to enhance the interaction as a way to treat diabetes. They created a molecular glue that stuck Ras and PI3Kα together. In animals, the compound lowered blood glucose levels (Science 2025, DOI: 10.1126/science.adr9097).
McCormick has a long-standing collaboration with Daiichi Sankyo, and when the scientists there told him about the molecular glue, “I jumped on it and used it to solve the structure of the complex,” he says. McCormick says he wasn’t thinking about creating a breaker at that time. But when he and his colleagues saw that there was a cysteine on PI3Kα that binds to Ras, it became clear they could use that amino acid as a place to anchor a molecule that could prevent the protein-protein interaction.
Structure of BBO-10203.
That’s when BBOT got involved. The company developed a series of breaker molecules, eventually landing on BBO-10203 as their clinical candidate (Science 2025, DOI: 10.1126/science.adq2004).
“If you think of the glue as an open hand receiving Ras, we essentially turn that open hand into a fist that bounces Ras out,” BBOT’s Beltran says. BBO-10203 is currently in Phase 1 clinical trials.
BBOT wasn’t the only company thinking about developing a breaker. Vividion’s chief scientific officer Matt Patricelli says that Downward’s genetically engineered mice were truly compelling evidence for what breaking up the Ras-PI3Kα interaction can do.
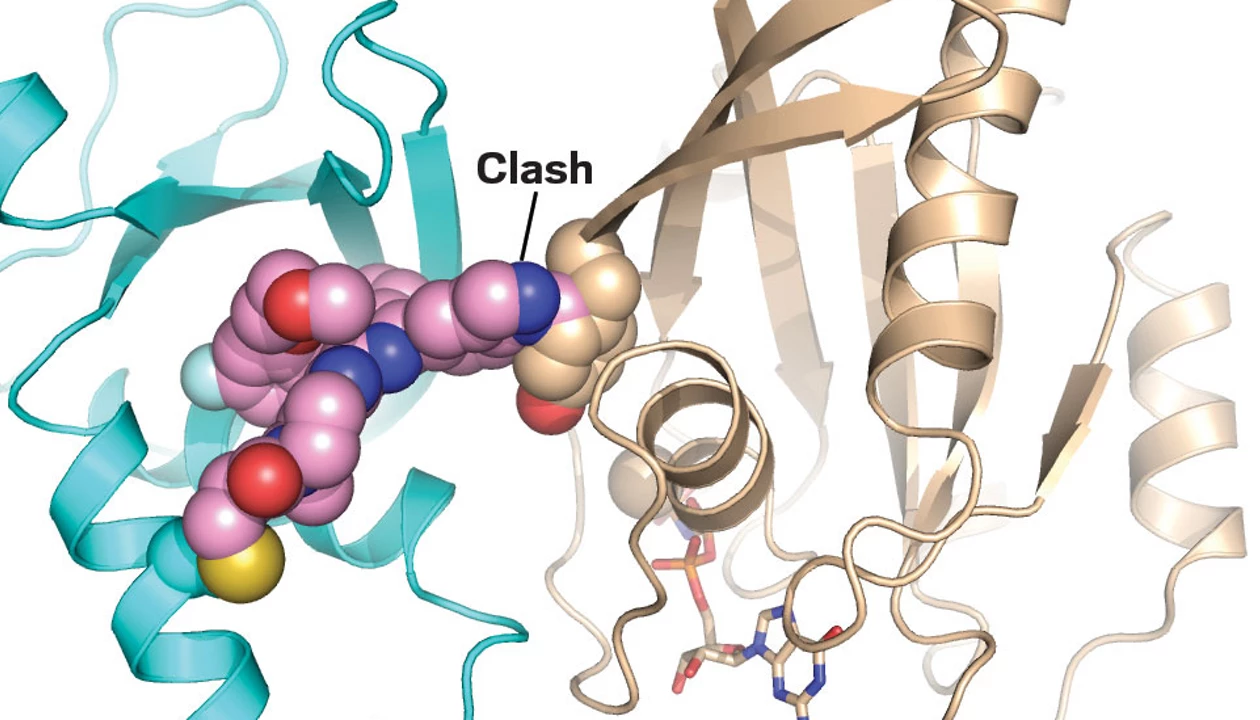
A small molecule is positioned between a protein ribbon structure labeled PI3Kα and a second ribbon structure positioned behind it and labeled Ras.
This model shows how VVD-442 stabilizes a conformation of PI3Kα that prevents Ras interaction.
Credit:
Vividion Therapeutics
To try to find a way to disrupt the Ras-PI3Kα interaction, Vividion scientists looked at covalent fragments in a chemoproteomics screening platform. Those fragments uncovered the same cysteine in PI3Kα’s Ras-binding domain that McCormick’s team had found and showed it was “primed to react with electrophiles,” Patricelli says. “It seemed like a fairly easy site to get selective reactions, which is usually what starts us down the path to trying to make a drug.”
Structure of VVD-442.
Last month, Vividion and Downward reported tool compounds, including VVD-442, which are Ras-PI3Kα breakers (Science 2025, DOI: 10.1126/science.adv2684). Unlike BBO-10203, which directly blocks the Ras-PI3Kα interaction, Vividion’s compounds induce a conformational change in PI3Kα that prevents interaction with Ras. The company also has a breaker compound in Phase 1 clinical trials—VVD-159642—although its structure has not been disclosed.
Patricelli says Vividion saw lackluster results when doing tests with their breakers in cells. “There was no effect on cell growth in culture, no matter what we did,” he says. But Downward’s animal models were so compelling that the company felt it had to move the compounds into animal models before deciding the program’s fate. That’s when the compounds’ potential became apparent.
Frontier also reported its own Ras-PI3Kα breaker, FMC-242, last month. All three companies say they envision using the breakers in combination with other cancer-fighting drugs, such as Ras inhibitors.
Bart Vanhaesebroeck, who studies PI3K signaling in cancer at University College London and is not involved in developing Ras-PI3Kα breakers, says that this is a clever strategy but that he isn’t sure how it will pan out clinically.
What Vanhaesebroeck did find intriguing was a surprise that turned up in both the BBOT and Vividion work: these Ras-PI3Kα breakers fight cancers driven by the protein human epidermal growth factor receptor 2 (Her2). “They have discovered, by serendipity, something else to interfere with Her2,” he says. This could be a boon for treating Her2-positive breast cancer, Vanhaesebroeck adds, although there’s some basic biology that needs to be worked out.
Patricelli says, “There’s not a known role for Ras in Her2 signaling,” so how the breakers are able to fight Her2 is something of a mystery. “This is where I think the fun part of this whole story, going forward, will be,” he says, because BBOT and McCormick disagree with Vividion and Downward on what is happening. The former think there’s an unknown Ras that’s being inhibited, but the latter don’t think that’s the case. “We have molecules that don’t disrupt the Ras interaction, but they still block the Her2 signaling,” Patricelli says.
McCormick agrees that it’s a mystery. “It’s complicated, but we’ll figure it out,” he says.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society