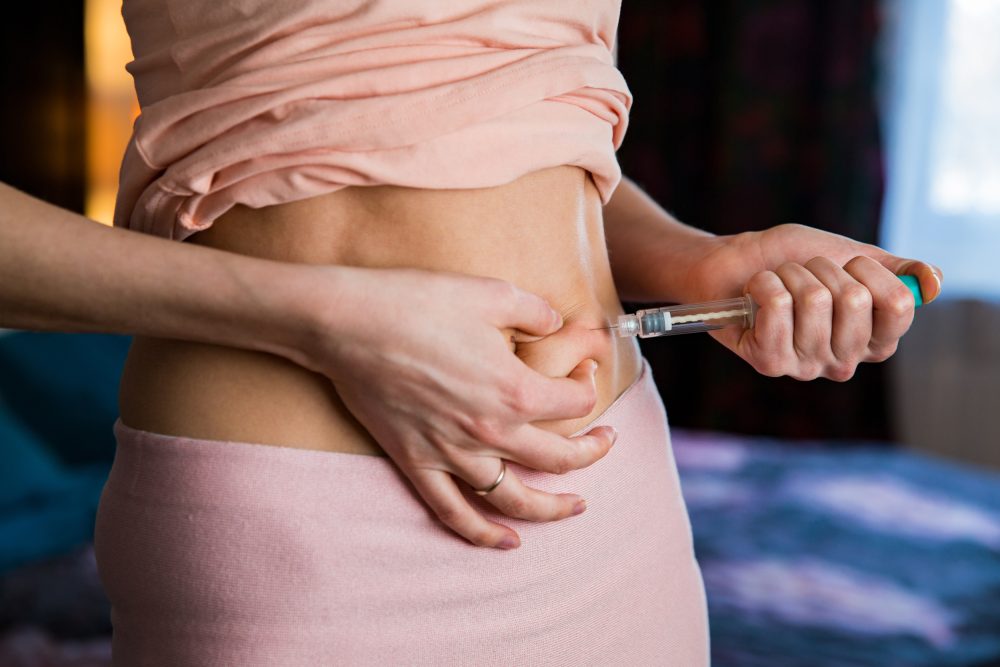
Birth control shots that people can use on their own have been available in the United States for a long time. However, many doctors still do not offer this option to patients.
A new study shows that many doctors do not even know this type of birth control exists.
This matters because self-injectable birth control can make life easier by giving people more privacy and control.
The study explains why this option is rarely offered in clinics and what changes could help more clinics offer it to patients across the country.
An overlooked birth control option
The researchers surveyed 422 clinicians in the United States who regularly prescribe birth control. Only about one third of clinicians knew about self-injectable contraception.
Among those who knew, only a portion actually prescribed it. Overall, only around a quarter of reproductive health experts offered this method to patients.
Many clinicians shared similar concerns. Some worried about whether patients could inject the medication correctly.
Others pointed to problems such as limited availability at pharmacies or a lack of clear guidance on how to counsel patients. Time pressure during appointments also played a role.
The study marks the first time experts clearly documented barriers that prevent wider use of self-administered injectable contraception.
Awareness shapes access
Dr. Jennifer Karlin, an associate professor of family and community medicine at UCSF, is the study’s senior author.
“Since most physicians don’t know that this is an option, patients don’t know about it,” said Dr. Karlin.
“It’s safe, effective, and puts the control in patients’ own hands. We should be talking about and offering it to patients without biases.”
When doctors do not mention certain options, patients miss chances to choose what best fits their lives and comfort.
Self-injection can reduce clinic visits and help people manage birth control on personal schedules.
How injectable contraception works
The injectable contraceptive used in this study is called depot medroxyprogesterone acetate, also known as DMPA.
The contraceptive contains progestin, a synthetic form of the hormone progesterone. This hormone plays a key role in pregnancy prevention.
DMPA works in three main ways. First, it stops ovulation so ovaries do not release eggs. Second, it thickens cervical mucus, which makes it harder for sperm to reach an egg.
Third, it thins the uterine lining, reducing the chance of pregnancy. One injection prevents pregnancy for up to three months.
Two forms of DMPA exist. One version, sold as Depo Provera, goes into a muscle and requires a clinician to administer it. The second version goes just under the skin.
This subcutaneous version allows safe self-injection, similar to injectable weight loss medications used today.
Safety and side effects
Like all medications, DMPA comes with possible side effects. Some users experience weight gain or reduced bone mineral density.
Research also links the drug to a rare and benign brain tumor called meningioma. The overall risk remains low, but clear discussion remains important.
Clinicians should explain both benefits and risks so patients can make informed decisions.
Open conversations help build trust and ensure people understand how the medication may affect health over time.
Usage across countries
The subcutaneous version of DMPA received approval in 2004.
Official labeling still lists clinician administration, yet doctors have trained patients to self-inject safely for many years.
Outside the United States, self-injectable contraception sees wider use, especially in sub Saharan Africa.
Use increased in the United States during the COVID-19 pandemic. Many clinics reduced in person visits, which pushed interest in home based care.
More than half of clinicians in the study learned about self-injection between 2020 and 2022.
Barriers and possible solutions
Even though national and international guidelines support access for all patients, gaps remain.
Clinicians in states with restricted abortion access prescribed self-injectable contraception less often. Other barriers include limited educational materials, staffing challenges, and short appointment times.
The authors recommend a broad education campaign for clinicians. Training should focus on counseling, prescribing, and teaching safe injection techniques.
The researchers also support FDA approval for official self-administration labeling, better insurance coverage, and simpler clinic workflows so more patients can easily access this birth control option without extra barriers.
With these changes, self-injectable contraception could become a regular option rather than a hidden one.
The study is published in the journal Obstetrics and Gynecology.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–