On December 30, Perth woman Kerry Smith felt a scratchy throat coming on and decided to take ArmaForce, a complementary medicine that promotes boosted immunity and relief from cold and flu symptoms, which she had taken before with no ill effects.
“Within 10 minutes, I started to feel my palms and my feet start to itch,” Ms Smith told Damian Smith on ABC Radio Perth.
“I went back to the bathroom and said to my husband, ‘I’m having a reaction, I’ve taken this [Armaforce]’.
“Then I collapsed on the floor with excruciating stomach cramps, and then shortly after that, I started to have difficulty breathing and my tongue swelled up.
“It all happened in the space of about 5 minutes. It was very, very fast and very, very scary.”
Her husband called an ambulance and administered an EpiPen, which is used to self-inject adrenaline to quickly treat severe allergic reactions, before the ambulance arrived.
Ms Smith had EpiPens in the house because she had a history of allergy to bee stings, but had rarely needed to use them.
“The emergency doctors at the hospital said that if we didn’t have the EpiPen on hand, that the outcome could have been much more serious,” she said.
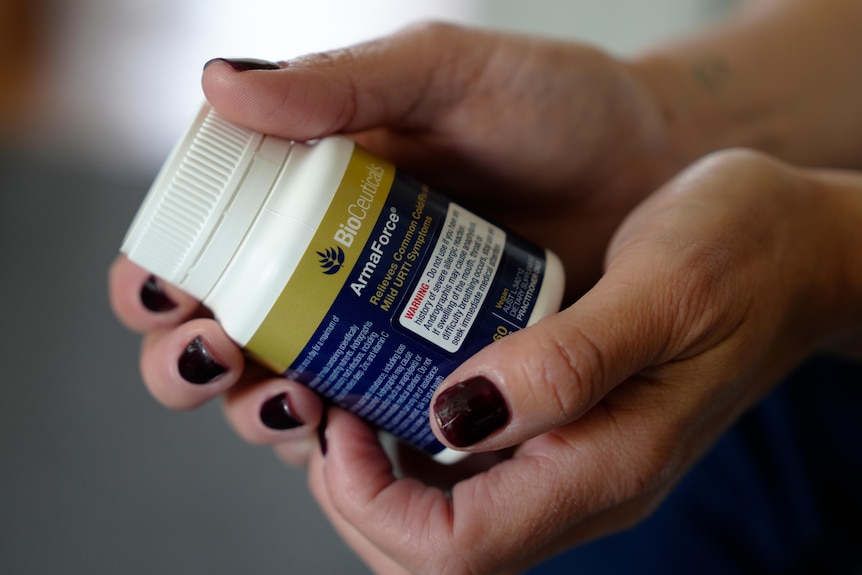
ArmaForce is a complementary medicine that promotes boosted immunity. (ABC News: Jerry Rickard)
When she told the doctors at the emergency department what she had taken, they immediately recognised an adverse reaction to a herb called Andrographis paniculata, an ingredient in ArmaForce.
“They all said, ‘Oh, we’ve had so many presentations of people having a similar reaction’.
“But I’d never heard of that herb before.”
Multiple safety reviews
While many members of the public have never heard of Andrographis paniculata, it has been on the radar of the Therapeutic Goods Administration (TGA) since 2008, when it first reviewed the medication “in response to adverse event reports and community concerns about anaphylaxis,” it said in a statement.
Since that time, several safety alerts have been issued, and in 2019, a label warning requirement was introduced for all products containing Andrographis.
“Despite this requirement for a warning label, adverse event reports to the TGA have continued to increase, with a fatal case of anaphylaxis reported in 2024,” the TGA statement said.
“To date, the TGA has received 1,365 adverse event reports for medicines containing Andrographis paniculata, including 859 for ArmaForce products. There have been a total 286 reports of anaphylaxis for medicines containing Andrographis paniculata, including 188 reports involving ArmaForce products.”

There is a warning label on ArmaForce bottles. (ABC News: Jerry Rickard)
BioCeuticals, the company that sells ArmaForce, said in a statement they “empathise with anyone who has experienced a suspected adverse reaction associated with any of our products”.
“Products containing andrographis may cause allergic reactions in some people, including in rare cases anaphylaxis. All required warnings relating to this risk are included on current product packaging in line with TGA requirements.
“To further strengthen consumer awareness, in 2024 BioCeuticals introduced front-of-pack boxed warning labels.”
Warning confusion
Ms Smith said that while she read the label, she misunderstood it to mean that she should not use it if she had a history of severe allergic reaction to ArmaForce, which she had previously taken without incident.
In fact, it means that anyone with a history of severe allergic reactions to anything, including bee stings, should not take the supplement.
“When I bought it and I saw the warning, I spoke to the pharmacist and the pharmacist only asked me two questions. They said, ‘Have you taken it before? And don’t take it for more than two weeks,'” she said.
“I wasn’t asked about my allergy history, and it definitely wasn’t brought to my attention.”
Kerry Smith continued to experience symptoms after she was taken to hospital, including swelling and hives. (Supplied: Kerry Smith)
Cause of reactions unclear
Australasian Society of Clinical Immunology and Allergy president Michael O’Sullivan said Ms Smith could not have been expected to know she would have a reaction.
“It really doesn’t matter if you’ve had a reaction to a bee sting or something else before, anyone could be at risk of having a reaction to a medication, including anaphylaxis,” Dr O’Sullivan said.
“It could have just as easily happened to her whether or not she had the bee sting allergy.”

Michael O’Sullivan is an immunologist at Perth Children’s Hospital. (Supplied)
Dr O’Sullivan said Ms Smith’s experience was a cautionary tale that showed over-the-counter complementary medicines could carry the same risks of side effects as prescription medication.
“Herbal supplements and these over-the-counter remedies that a lot of people might not think of carrying the same risk of side effects and reactions as something like taking an antibiotic that your doctor’s prescribed,” he said.
“But we know that they can have side effects, including allergic reactions, and interfere with other medications or cause other adverse drug reactions.”
He said it was unclear why Andrographis was associated with such a high number of allergic reactions compared to other herbal remedies, and patients needed to weigh up the risks.
“It’s obviously an individual decision and we don’t want to overstate the risk — there will be thousands of people who take these medications and these supplements with no problems,” he said.
“But ultimately, if we had proven effective medications for common viral infections, then we would be recommending them.
“When you see a GP, you’d be getting prescribed them or recommended to go and buy them, and that evidence just doesn’t exist for a lot of these complementary medicines. That’s why they’re complementary medicines.”
After spending a night in hospital, Ms Smith has made a full recovery.

