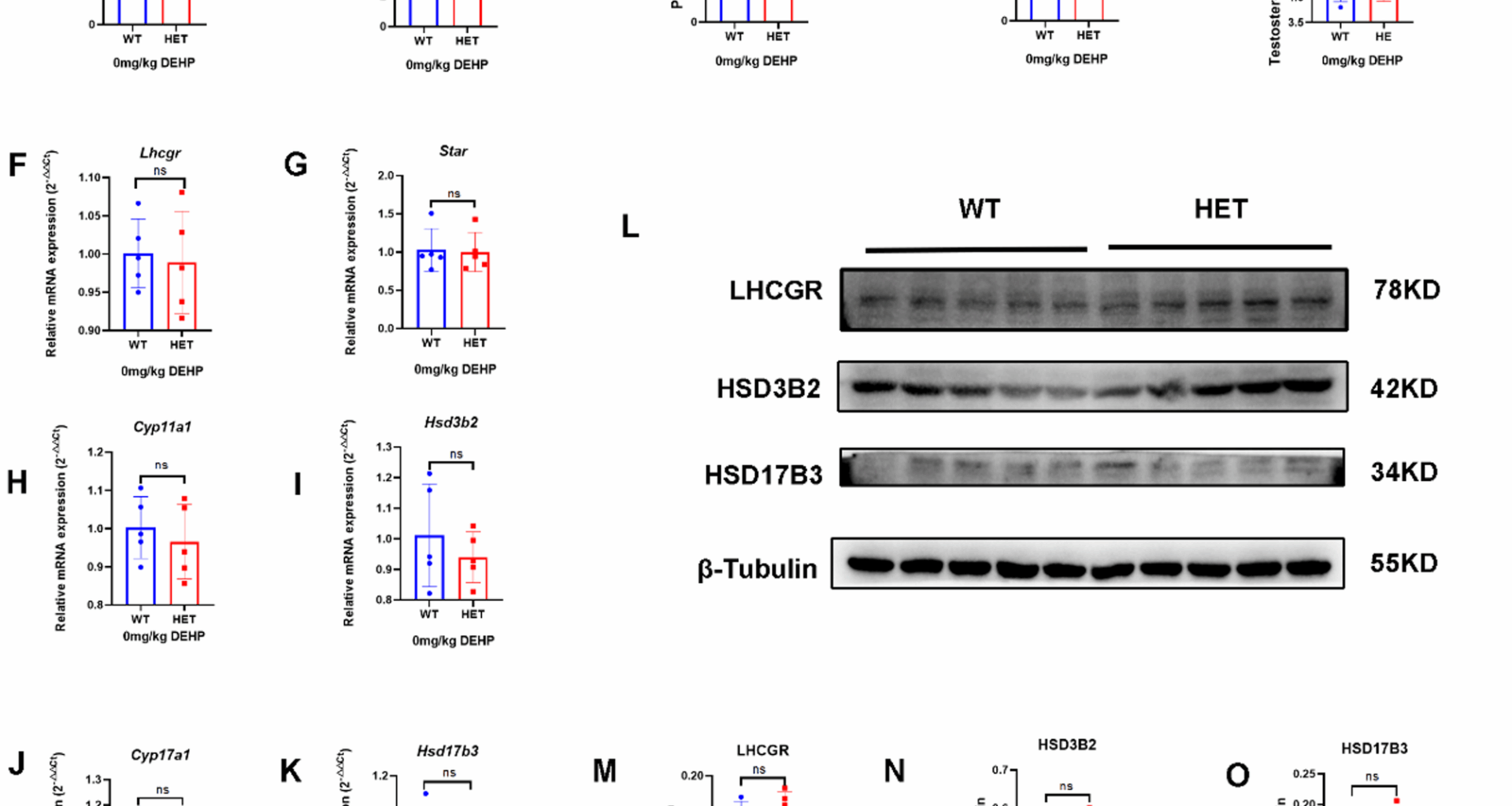
LhcgrW495X/+ male offspring showed normal phenotype and steroidogenic gene expressionLhcgrW495X/+ male offspring showed normal phenotype and steroidogenic gene expression in the neonatal stage
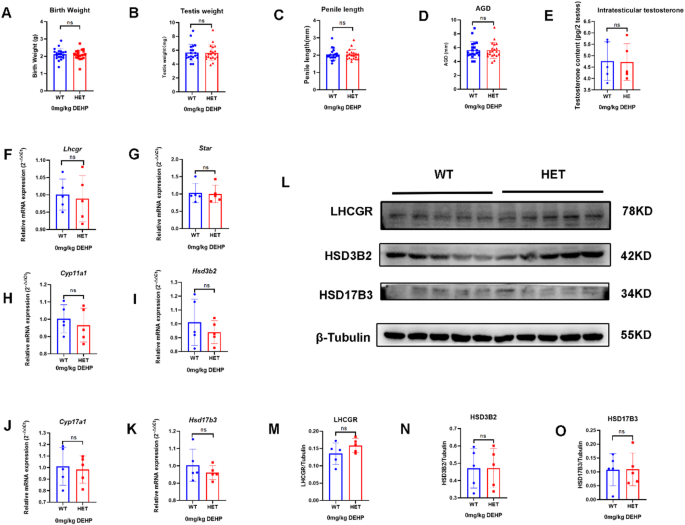
In the neonatal stage, no DSD (hypospadias or micropenis) was found in the WT and HET groups. The two groups had no significant differences in birth weight, testis weight, penis length, AGI, and intratesticular testosterone (Fig. 1A-E). There was no significant difference in mRNA expression of steroidogenic genes (Lhcgr, Star, Cyp11a1, Hsd3b2, Cyp17a1, and Hsd17b3) in the neonatal mice between the two groups (n = 5, p > 0.05) (Fig. 1F-K). WB analysis also confirmed no significant differences between the two groups in the expression of LHCGR, HSD3B2, and HSD17B3 (Fig. 1L-O). Therefore, LhcgrW495X/+ male offspring showed normal phenotype and steroidogenic gene expression in the neonatal stage.
LhcgrW495X/+ male mice showed normal phenotype and steroidogenic gene expression in the neonatal stage. A-E: there was no significant difference in birth weight, testis index, penile length, AGI, and intratesticular testosterone between WT and HET groups. F-K: there was no significant difference in mRNA expression of steroidogenic genes (Lhcgr, Star, Cyp11a1, Hsd3b2, Cyp17a1, and Hsd17b3) between WT and HET groups. L-O: there was no significant difference in LHCGR, HSD3B2, and HSD17B3 expressions between WT and HET groups. ns: no significance
LhcgrW495X/+ male offspring showed normal phenotype and steroidogenic gene expression in the adult stage
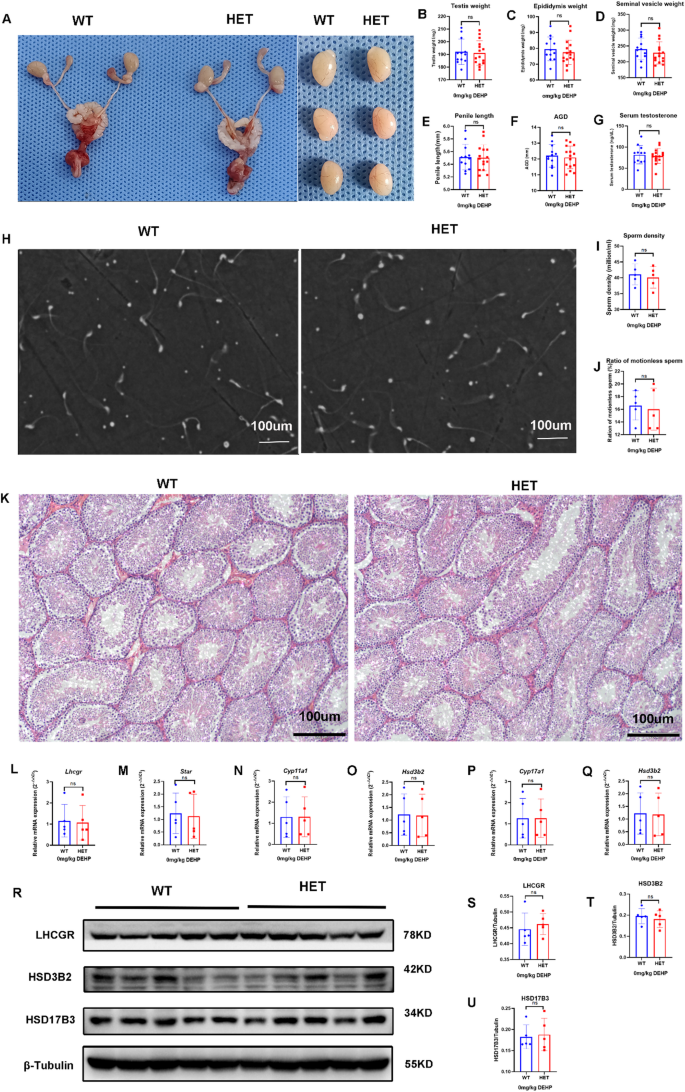
In the adult stage, genital development, including that of the testis, was normal, and no DSD was found in both groups (Fig. 2A-F). There were no significant differences in serum testosterone, semen quality (sperm density and proportion of immotile sperm), and seminiferous tubule morphology between the two groups (Fig. 2G-K). These results suggest that LhcgrW495X/+ male offspring showed normal phenotype in the adult stage.
There was no significant difference in the mRNA expression of steroidogenic genes (Lhcgr, Star, Cyp11a1, Hsd3b2, Cyp17a1, and Hsd17b3) in adult offspring between the two groups (n = 5, p > 0.05) (Fig. 2L-Q). WB analysis also confirmed that there were no significant differences in the expression of LHCGR, HSD3B2, and HSD17B3 (n = 5, p > 0.05) (Fig. 2R-U). These results suggested that LhcgrW495X/+ male offspring showed normal steroidogenic gene expression in the adult stage.
LhcgrW495X/+ male mice showed normal phenotype and steroidogenic gene expression in the adult stage. A-G: there was no difference in genital development and serum testosterone between the two groups. H-J: there was no significant difference in semen quality between the two groups (X100). K: HE staining (X100) of testicular tissue showed no significant difference in the development of seminiferous tubules between the two groups. L-Q: the two groups showed no difference in mRNA expression of steroidogenic genes (Lhcgr, Star, Cyp11a1, Hsd3b2, Cyp17a1, and Hsd17b3). R-U: the two groups showed no difference in expressions of LHCGR, HSD3B2, and HSD17B3. ns: no significance
Prenatal exposure to low-dose DEHP selectively induced DSD in LhcgrW495X/+ male offspring by interfering with steroidogenic gene expressionPrenatal exposure to low-dose DEHP inhibited testosterone synthesis in LhcgrW495X/+ neonatal male offspring by interfering with steroidogenic gene expression
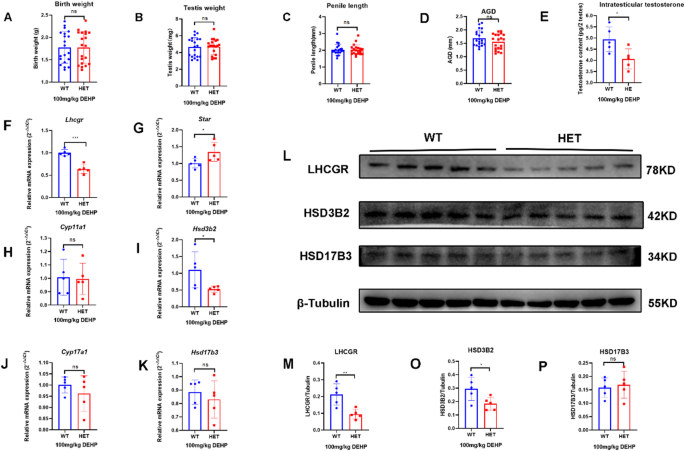
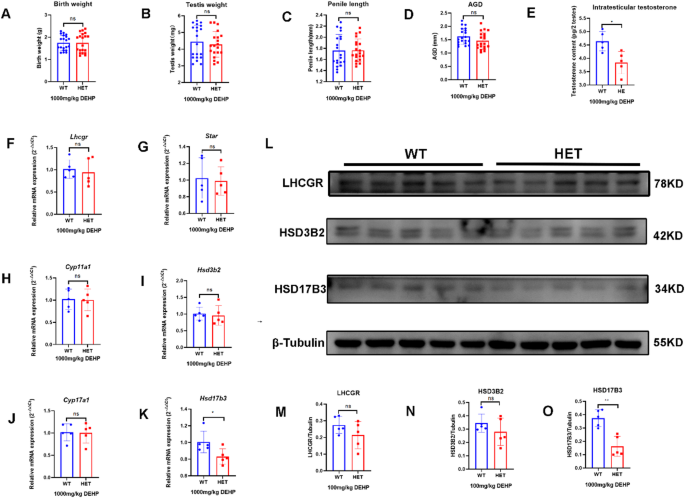
After prenatal exposure to low-dose (100 mg/kg/d) DEHP, no DSD was observed in the two groups in the neonatal stage. There were no significant differences in birth weight, testis weight, and penis length in neonatal male mice between the two groups. However, the AGD and intratesticular testosterone in the HET group were lower than those in the WT group (Fig. 3A-E). The results suggested that prenatal DEHP exposure affected genital development and testosterone synthesis in LhcgrW495X/+ male offspring (Fig. 3A-E).
There was no significant difference in mRNA expression of Star, Cyp11a1, Cyp17a1, and Hsd17b3 in neonatal offspring between the two groups (n = 5, p > 0.05). mRNA expression of Lhcgr and Hsd3b2 in the HET group were lower than that in the WT group (n = 5, p < 0.05) (Fig. 3F-K). LHCGR and HSD3B2 expressions in the HET group were also lower than those in the WT group (n = 5, p < 0.05) (Fig. 3L-P). Because neonatal offspring were recently exposed to DEHP in utero, this experiment was similar to the cell experiment. These results indicated that prenatal DEHP exposure synergistically inhibited testosterone synthesis in LhcgrW495X/+ neonatal male offspring by interfering with steroidogenic gene expression.
Prenatal exposure to low-dose DEHP inhibited testosterone synthesis in LhcgrW495X/+ neonatal male mice by interfering with steroidogenic gene expression. A-E: the HET group showed reduced AGI and intratesticular testosterone. F-K: the mRNA expressions of Lhcgr and Hsd3b2 in the HET group were lower than in the WT group. L-P: The expressions of LHCGR and HSD3B2 in the HET group were lower than in the WT group. ns: no significance; * p < 0.05; ** p < 0.01; *** p < 0.001
Prenatal exposure to low-dose selectively DEHP selectively induced DSD and testicular dysfunction by interfering with steroidogenic gene expression in LhcgrW495X/+ adult male offspring
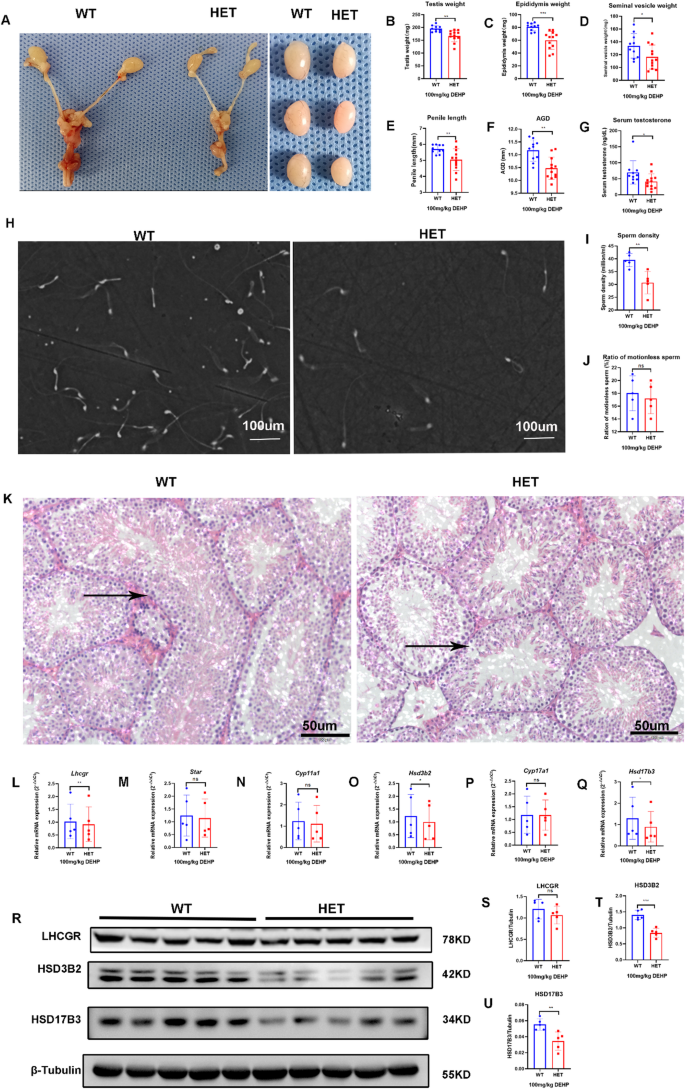
This study found that the weight of the unilateral testis in WT mice without DEHP exposure was > 85 mg. If the testis was poorly developed, all the genital organs (epididymis, seminal vesicle, and prostate) were poorly developed. Therefore, if the weight of the unilateral testis was < 85 mg, it was categorized as DSD in this study. After prenatal exposure to low-dose DEHP, the situation changed. DSD was found only in the HET group in the adult stage. The incidence of DSD in the HET group (41.18%) was significantly higher than that in the WT group (0%, p = 0.04). Genital development, manifested as reduced testis weight, epididymis weight, seminal vesicle weight, penis length, and AGI, in the HET group was worse than that in the WT group (Fig. 4A-F). Serum testosterone (Fig. 4G) and semen quality (sperm density and proportion of immotile sperm, Fig. 4H-J) in the HET group were lower than those in the WT group. The seminiferous tubule in the HET group showed poorer development, which demonstrated as reduced tubular diameter and length, and dilatation of the lumen (Fig. 4K). These results suggest that prenatal exposure to low-dose DEHP synergistically induced DSD and testicular dysfunction (low testosterone and poor sperm quality) in LhcgrW495X/+ adult male offspring. WT offspring showed nearly normal genital development and greater tolerance to prenatal DEHP exposure.
Although mRNA expression of Star, Cyp11a1, and Cyp17a1 showed no difference, mRNA expression of Lhcgr, Hsd3b2, and Hsd17b3 in the HET group were lower than that in the WT group (n = 5, p < 0.05) (Fig. 4L-Q). WB also confirmed that the expression of LHCGR, HSD3B2, and HSD17B3 was lower in the HET group than that in the WT group (n = 5, p < 0.05) (Fig. 4R-U). These results show the synergistic effect of prenatal DEHP exposure on steroidogenic gene expression in LhcgrW495X/+ adult male offspring.
Prenatal exposure to low-dose DEHP induced DSD and testicular dysfunction by interfering with steroidogenic gene expression in LhcgrW495X/+ adult male mice. A-G: LhcgrW495X/+ adult male mice showed DSD, poorer genital development, and lower serum testosterone. H-J: LhcgrW495X/+ adult male mice showed poorer semen quality (X100). K: HE staining (X200) of testicular tissue (→) showed poorer development of seminiferous tubules in the HET group. L-Q: The mRNA expressions of Lhcgr, Hsd3b2, and Hsd17b3 in the HET group were lower than that in the WT group. R-U: LHCGR, HSD3B2, and HSD17B3 expressions in the HET group were lower than in the WT group. ns: no significance. * p < 0.05; ** p < 0.01; *** p < 0.001
Prenatal exposure to high-dose DEHP synergistically exacerbated DSD in LhcgrW495X/+ male offspring by interfering with steroidogenic gene expressionPrenatal exposure to high-dose DEHP inhibited testosterone synthesis by interfering with steroidogenic gene expression in LhcgrW495X/+ neonatal male offspring
After prenatal exposure to high-dose DEHP, there was no significant difference in birth weight, testis weight, and penis length between the two groups. However, the AGI and intratesticular testosterone in the HET group were lower than those in the WT group (Fig. 5A-E). Previous studies had verified that prenatal exposure to high-dose DEHP inhibited genital development in WT mice [29, 30]. Thus, our results indicated that prenatal exposure to high-dose DEHP affected genital development and testosterone synthesis in WT and HET male offspring. However, developmental stunting was relatively more severe in HET male offspring.
There was no difference in the mRNA expression of Lhcgr, Star, Cyp11a1, Cyp17a1, and Hsd3b2 in neonatal male offspring between the two groups (n = 5, p > 0.05). However, the mRNA expression of Hsd17b3 in the HET group was lower than that in the WT group (n = 5, p < 0.05) (Fig. 5F-K). The expression of HSD17B3 in neonatal male mice in the HET group was also lower than that in the WT group (n = 5, p < 0.05) (Fig. 5L-O). These results further verified prenatal DEHP exposure synergistically inhibits testosterone synthesis by interfering with steroidogenic gene expression in LhcgrW495X/+ offspring.
Prenatal exposure to high-dose DEHP inhibited testosterone synthesis by interfering with steroidogenic gene expression in LhcgrW495X/+ neonatal male mice. A-E: the HET group showed reduced AGI and intratesticular testosterone. F-K: The mRNA expression of Hsd17b3 in the HET group was lower than in the WT group. L-O: The expression of HSD17B3 in the HET group was lower than in the WT group. ns: no significance; * p < 0.05; ** p < 0.01; *** p < 0.001
Prenatal exposure to high-dose DEHP synergistically exacerbated DSD and testicular dysfunction in LhcgrW495X/+ adult male offspring by interfering with steroidogenic gene expression
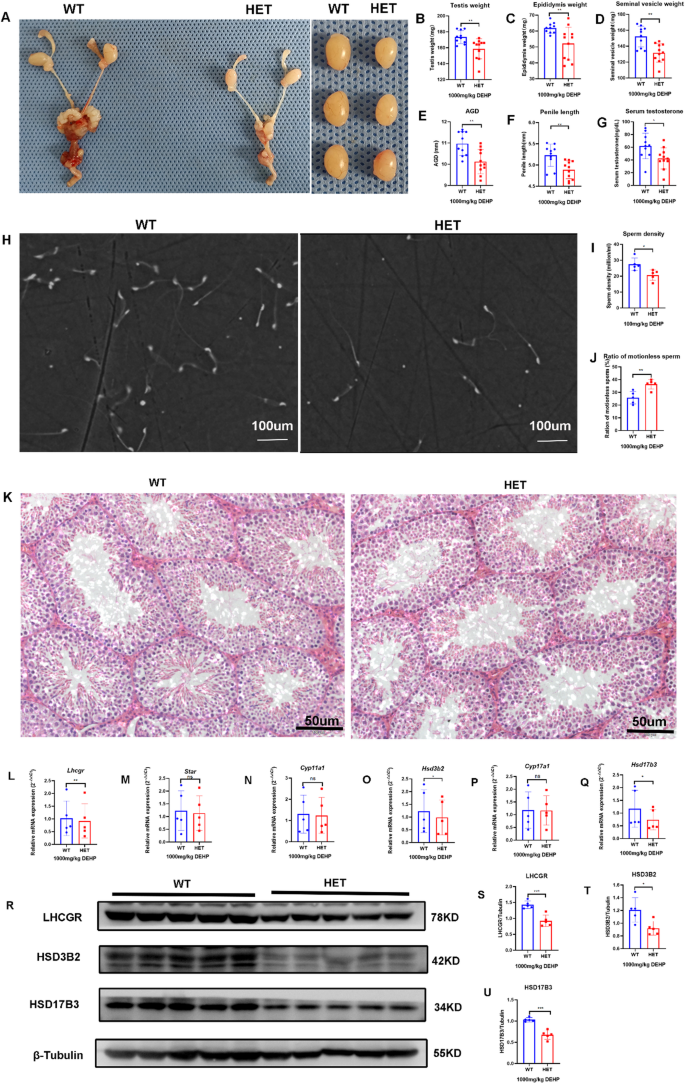
After prenatal exposure to high-dose DEHP, DSD was observed in both groups in adult offspring, but the incidence of DSD in the HET group (66.7%) was significantly higher than that in the WT group (16.7%, p = 0.03). Genital development in the HET group was also worse than that in the WT group, which manifested as reduced testis weight, epididymis weight, seminal vesicle weight, penis length, and AGI (Fig. 6A-F). Serum testosterone (Fig. 6G) and semen quality (Fig. 6H-J) were lower in the HET group than those in the WT group. The seminiferous tubule was shorter, and the internal diameter of the seminiferous tubule was wider in the HET group compared to those in the WT group (Fig. 6K). These results showed that prenatal exposure to high-dose DEHP induced DSD and testicular dysfunction in both groups. However, DSD was more severe in the HET group, thus further verifying that prenatal DEHP exposure synergistically aggravates DSD and testicular dysfunction in LhcgrW495X/+ adult male offspring.
Although the mRNA expression of Star, Cyp11a1, and Cyp17a1 showed no difference, the mRNA expression of Lhcgr, Hsd3b2, and Hsd17b3 were lower in the HET group than that in the WT group (n = 5, P < 0.05) (Fig. 6L-Q). WB also confirmed that the expression of LHCGR, HSD3B2, and HSD17B3 in the HET group were lower than that in the WT group (n = 5, p < 0.05) (Fig. 6R-U). These results further verified that prenatal DEHP exposure synergistically induces DSD by interfering with steroidogenic gene expression in LhcgrW495X/+ adult male offspring. It also suggested that the exposure dose impacted the synergistic effect on the interference with steroidogenic gene expression.
Prenatal exposure to high-dose DEHP synergistically aggravated DSD and testicular dysfunction in LhcgrW495X/+ adult male mice by interfering with steroidogenic gene expression. A-G: the genital development and serum testosterone were worse in the HET group (X100). H-J: the semen quality was poorer in the HET group. K: HE staining (X200) of testicular tissue (→) showed that the development of seminiferous tubules was poorer in the HET group. L-Q: The mRNA expressions of Lhcgr, Hsd3b2, and Hsd17b3 in the HET group were lower than that in the WT group. R-U: LHCGR, HSD3B2, and HSD17B3 expressions in the HET group were lower than in the WT group. ns: no significance; * p < 0.05; ** p < 0.01; *** p < 0.001