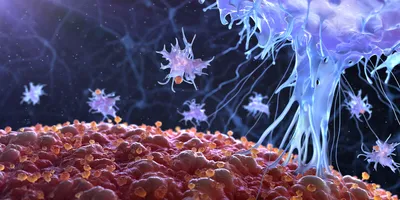
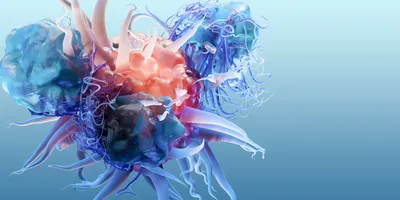
Once administered, the vector instructs the body to express these viral fragments, effectively teaching T cells what to look for.
istock.com/magicmine
Papzimeos is the first FDA-approved therapy for recurrent respiratory papillomatosis, a rare HPV-driven disease that has long forced patients into repeated surgeries.
Recurrent respiratory papillomatosis (RRP) is a rare disease caused by chronic infection with human papillomavirus (HPV) types 6 or 11, which leads to the growth of wart-like tumors along the respiratory tract.
These benign growths can block the airway, cause hoarseness, and trigger repeated respiratory complications, sometimes requiring dozens of surgeries over a patient’s lifetime. Though considered uncommon, RRP affects an estimated 27,000 adults in the United States, with each case carrying a heavy toll on quality of life and health care resources.
For adults living with RRP, surgery has long been the only way to keep breathing passages clear. But now, with the FDA approval of Precigen’s Papzimeos (zopapogene imadenovec-drba), patients finally have a treatment that goes beyond symptom control, offering the first therapy designed to target the disease at its source.
A new kind of therapy
Papzimeos takes a different approach. Instead of cutting away papillomas, it aims to train the immune system to recognize and destroy the HPV-infected cells that cause them.
Continue reading below…
The therapy uses a non-replicating adenoviral vector — part of Precigen’s proprietary AdenoVerse platform — to deliver a fusion antigen composed of selected fragments from HPV 6 and 11 proteins. Once administered, the vector instructs the body to express these viral fragments, effectively teaching T cells what to look for. In clinical testing, patients who responded to Papzimeos showed a significant expansion of HPV-specific T cells, confirming that the treatment achieved its intended immune reprogramming.
“This is not just symptom management, it’s the first therapy designed to address the root cause of RRP,” said Helen Sabzevari, CEO of Precigen, in the approval announcement.
Short-course dosing, long-lasting effect
Another defining feature of Papzimeos is its dosing schedule. The therapy is given as four subcutaneous injections over 12 weeks. Unlike surgery, which must be repeated indefinitely, the immunotherapy is designed to produce durable immune control long after the last shot.
In a study conducted at the National Institutes of Health, more than half of the 35 participants achieved complete response, meaning they required no surgeries in the 12 months after treatment.
Continue reading below…
Among those followed for two years, most remained papilloma-free, suggesting long-term immune memory. For patients accustomed to frequent operations, the prospect of sustained remission from just a few injections represents a dramatic shift.
The study also found Papzimeos to be well tolerated. No dose-limiting toxicities were observed, and no treatment-related adverse events exceeded Grade 2. The most common side effects were those typically associated with immune activation, mild injection site reactions, fever, chills, and fatigue.
The FDA label includes guidance for monitoring after the first injection and notes rare risks, such as thrombotic events, but overall the therapy’s safety profile compares favorably to the morbidity associated with repeated surgical intervention.
Why this approval matters
Several aspects make Papzimeos stand out:
Mechanistic precision: By targeting HPV 6 and 11 proteins, the therapy acts directly on the driver of RRP rather than its symptoms.Vector design: Non-replicating adenoviral delivery is well suited for eliciting strong T-cell responses, a key need in viral-driven papillomas.Durability: Evidence of sustained responses beyond 12 and 24 months indicates the potential for long-term control after a finite regimen.Practical benefit: A short course of injections could spare patients countless surgeries, reducing both health system burden and personal disruption.
The FDA’s decision to grant full approval, without requiring a confirmatory trial, also highlights the significance of the data. For a rare disease with limited options, regulators accepted single-arm trial results showing robust efficacy and biologic plausibility.
Continue reading below…
For the RRP community, the approval signals long-awaited progress in treatment options.
“For the first time, adult patients with RRP have access to an FDA-approved therapy that offers the potential to reduce, or even eliminate, endless repeated surgeries,” Kim McClellan, President of the Recurrent Respiratory Papillomatosis Foundation, said in a statement.
Precigen has launched Papzimeos SUPPORT, a program to help patients access the treatment through insurance navigation and financial assistance. With the therapy already cleared for broad use in adults, rollout is expected to begin immediately.