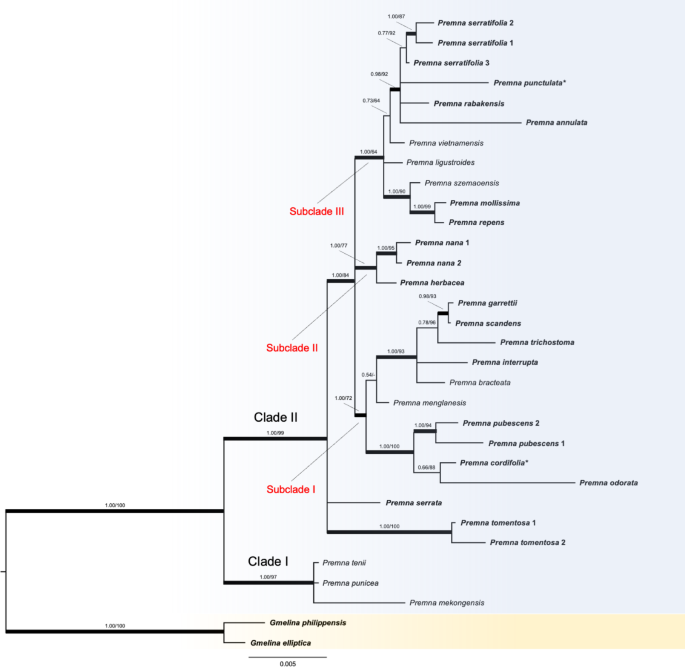
Phylogenetic reconstruction
By using multi-locus approach of four chloroplast markers, this study provided the first phylogenetic relationships with emphasis on species of Premna in Thailand (bolded species, Fig. 1). Our findings are strongly consistent with previous studies based on chloroplast regions and plastome analyses, confirming that the genus Premna is a monophyletic within the subfamily Premnoideae and forms a sister relationship with Gmelina L.5,6,17,20,32,41,42. However, the phylogenetic positions of some species remain unresolved due to polytomy, such as P. ligustroides, P. serrata, and P. tomentosa. These unresolved relationships have been commonly observed in previous phylogenetic analyses within Premna5,17 and other taxa in many Lamiaceae genera, such as Leucas R.Br., Otostegia Benth., Phlomis L., Phlomoides, Salvia L., Stachys L.37,43,44,45. This may be the result of insufficient data, as few chloroplast markers have been used. Therefore, further studies employing more comprehensive approaches, such as plastid genome, a target enrichment or whole genome analyses, are necessary to explore these relationships (e.g. Zhao et al.6,46, Satthaphorn et al.47). In this study, phylogenetic relationships based on four concatenated chloroplast markers resolved in two clades (Fig. 1). In following, phylogenetic positions and morphological characteristics are discussed.
CLADE I—This highly supported clade includes species distributed in China and Vietnam, namely P. mekongensis, P. punicea, and P. tenii, which are characterized by morphological synapomorphies such as cymes in a dense capitate inflorescence and calyces distinctly 5-lobed. This phylogenetic placement is consistent with morphology-based classification48. Further molecular study is required to test whether all species with the similar traits as recorded in Flora of China49 and adjacent regions form a monophyly.
CLADE II —The well-supported clade consists of a clade comprising subclades I to III (red labelled in Fig. 1), with the unresolved relationships among P. serrata and P. tomentosa. These two latter species possess distinct morphological characteristics from all species in Thailand. Premna serrata has serrate leaf margin and distinct calyx lobes (1–1.8 mm long)8,28. Premna tomentosa, previously recognized as P. pyramidata by Chen and Gilbert49 and Leeratiwong et al.8, exhibits stellate or dendritic hairs on both leaf surfaces, a unique trait distinguishing from other Premna (vs. non-stellate or dendritic in other species). The presence of this characteristic has been documented in several morphological studies3,4,8,22,49. In addition, this clade incorporated several species that have been not included in previous phylogenetic studies, such as P. annulata H. R. Fletcher, P. cordifolia, P. repens H. R. Fletcher, P. scandens Roxb., P. serrata, P. tenii, P. tomentosa, and P. trichostoma Miq.
Three unresolved subclades (subclades I to III) within Clade II were identified based on phylogenetic positions (with high ML and full BS supports, Fig. 1) and morphological characteristics. These subclades include several species in Thailand, as well as members of the P. serratifolia complex in Thailand. Although clear morphological characteristics have been not identified to support the assignment of infrageneric ranks across all subclades, we observed certain habits and morphological affinities among particular species to discuss with their phylogenetic positions.
Subclade I includes nine species of Premna which are shrub or woody climber, distributed in Myanmar, China, and Thailand. These species are mostly inhibited in hill evergreen or limestone forests8. Of these, five species forming a monophyletic group within this subclade (P. bracteata Wall. ex C. B. Clarke, P. garrettii H. R. Fletcher, P. interrupta, P. scandens, and P. trichostoma) have shared clavoid fruits and a thin, fleshy pericarp layer, whereas other species exhibit more or less globose fruits with thicker pericarp3,4,8,49,50. These fruit characteristics, along with endocarp morphology, have been validated as important diagnostic features for species identification and grouping of Premna in Thailand50 and align with the results of our chloroplast-based analysis. This subclade also involves P. cordifolia, a species though to be a synonym under P. serratifolia (subclade III) in the Malesian treatment3. The presence of P. cordifolia in this subclade, integrated with morphological evidence, greatly support the distinction between these taxa, as congruent with earlier treatments8,50,51. Additional samples for this subclade exhibiting the synapomorphy is necessary to further establish a robust foundation for a potential morphology-based infrageneric classification. Subclade II consists of two herbaceous species: P. herbacea and P. nana. Both species display a herbaceous suffrutescent habit with a woody rootstalk and softly woody to herbaceous stems which can be found in dry places, particularly in deciduous forest across southeast Asia8,49,52.The close relationships of these two species are consistent with the previous phylogenetic analyses by Li et al.5 and Hai et al.17. Premna nana was treated as a synonym of P. herbacea in the revision by Chen and Gilbert49. However, subsequent studies and our findings have supported the recognition of P. nana as a distinct species based on morphological characteristics (see identification key below) and molecular phylogenetic evidence3,4,5,8,17.
Subclade III contains Premna representatives which are diverse in their habits and morphological characteristics. Most species are shrubs, scandent shrubs, or woody climbers, with the exception of P. mollissima, P. rabakensis, and P. szemaoensis C. Pei, which are tree species. Species belonging to this clade occupy wide range of habitats such as coastal, evergreen, hill evergreen, or limestone forests8. Distinct morphological traits among species were observed such as the presence or absence of an interpetiolar ridge on stems, type of hairs on leaf surfaces, the size and shape of the calyx lobes. Some species within this subclade are also endemic, including P. annulata (Thailand), P. szemaoensis (China), and P. vietnamensis (Vietnam)8,17,49. Three accessions of P. serratifolia sampled from different sides of peninsular Thailand recovered a monophyletic group also present within this subclade (0.77/92) as well as its previously synonymized species, P. punctulata3. Our results showed that P. punctulata was unresolved with respect to P. serratifolia, exhibiting a relatively long branch, which reflects a greater degree of genetic change53. However, this pattern confirms that these taxa are not fully represented as genetically discrete entities54. This result is consistent with previous phylogenetic studies, which likewise reported a distinct phylogenetic position for P. punctulata separate from P. serratifolia5,17. Our findings thus reaffirm this separation, further supported by morphological and ecological evidence (see taxonomic note below).
Taxonomic notesNote on P. serratifolia complex in Thailand
As part of the preparation of Premna for the Flora of Thailand, the taxonomic status of three names, including P. cordifolia, P. paniculata, and P. punctulata, has remained ambiguous in relation to P. serratifolia, which were thought to be synonyms in the previous study3. However, our integrated morphological and ecological investigation, and chloroplast-based phylogenetic analyses support the recognition of these as distinct species, prompting a revision of their taxonomic identities (Table1, Fig. 1). Notably, P. serratifolia is confined to coastal habitats, whereas the other species are restricted to evergreen forests. The relevant taxonomic literature, comparative morphology and ecological descriptions, and taxonomic notes for each species are provided. A list of examined specimens is included in Supplementary File, with their mapped distributions shown in Fig. 6.
Premna cordifolia
Roxb., [Hort. Bengal.: 95. 1814, nom. nud.], Fl. Ind. 3: 78. 1832; Schauer in DC., Prodr. 11: 632. 1847; Miq., Fl. Ned. Ind. 2: 895. 1858; C. B. Clarke in Hook. f., Fl. Brit. India 4(12): 572. 1885; King & Gamble, J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 74: 818. 1909; H. J. Lam, Verbenaceae Malayan Archipel.: 111. 1919; Ridl., Fl. Malay Penins. 2: 621. 1923; Dop in Lecomte et al., Fl. Indo-Chine 4(7): 816. 1935; H. R. Fletcher, Bull. Misc. Inform. Kew 1938: 418. 1938; Kochummen in Ng, Tree Fl. Mal. 3: 307. 1978; Phuong in N.T. Ban et al., Fl. Vietnam 3: 298. 2005; Leerat. et al., Trop. Nat. Hist. 9(2): 119. 2009.—Gumira cordifolia (Roxb.) Kuntze, Rev. Gen. Pl., 2: 507. 1891. Type: Roxburgh illustration no. 1462 (lectotype K!, selected by Daniel & Rajendran55; isolectotype CAL!) (Fig. 2).
Premna cordifolia. (A, B) habit and habitat; (C) flowering branch; (D) inflorescence; (E) flowers; (F) fruits. Photos A, C–F by C. Leeratiwong and B by J. Satthaphorn.
Description: Scandent shrub or woody climber. Branches with an interpetiolar woody ridge, brown to reddish-brown pubescent. Leaves decussate, mostly cordate or cordate-ovate, rarely oblong-ovate, 5–15 by 3–7 cm, apex caudate or acuminate, rarely retuse, base cordate or rounded, margins entire; adaxial surface glabrous, shining, midrib pubescent; abaxial surface glabrous, with yellowish-brown or yellow sessile glands, midrib sparsely brown pubescent, nerve axils of lower half of abaxial leaf surface glabrous to sparsely pubescent; petiole 1–4 cm long. Inflorescence corymbose, 4–8 cm long; pedicels 0.5–1.5 mm long. Calyx pale green or green, (1.5–)2–3 mm long, unequally 2-lipped; tube 1.2–2.7 mm long; posterior lip entire or subentire; anterior lip 2-lobed, lobes 0.1–0.6 mm long. Corolla yellowish-white or white, 2-lipped, 5–7 mm long; tube 2–3.5 mm long, distally sparsely pubescent outside, densely white villous distally inside; posterior lip ovate to elliptic, 2.5–3.5 mm long; anterior lip with middle lobe slightly larger, broadly oblong or oblong-ovate, 1.2–1.5 mm long, with yellow patch on middle lobe; lobes with villose hairs on base. Stamens long exserted, filaments 1.5–3.5 mm long; anthers 0.3–0.6 mm long. Ovary ovoid to obovoid, 1–1.2 mm long, glabrous with sparse sessile glands at apex; stigma branches 0.15–0.25 mm long. Fruits black, broadly obovoid, 4–7 mm long, glabrous with sessile glands.
Distribution: Thailand (Songkhla, Yala, Narathiwat), Vietnam.
Ecology: In open areas or partly shaded primary or secondary evergreen forests, dry evergreen forests, hardwood forests, granite bedrock; elevation up to 380 m. Flowering and fruiting March to July.
Vernacular: ya yo (ยายอ).
Note: Premna cordifolia was considered as a ‘nomen nudum’ and listed as a synonym under P. serratifolia in de Kok’s3 treatment for Flora Malesiana. This corresponds to its first publication by Roxburgh56 in Hortus Bengalensis, which was invalid due to the absence of a description and type specimen. However, the name was later validated by Roxburgh57 in Flora Indica, although the origin of the type locality remains uncertain due to Roxburgh’s drawing was based on the cultivated specimen55. Therefore, P. cordifolia is a validly published and accepted name.
Although the previous treatment3 lacked the explanation for this inclusion, we presumed that specimens initially thought to be P. cordifolia (non Roxburgh) are referrable to P. serratifolia based on their similar morphological characteristics. However, our morphological investigation and ecological observations (Table 1) demonstrate that the original concept of P. cordifolia as published by Roxburgh57 is clearly distinct from P. serrtifolia. Specimens of P. cordifolia (sensu Roxburgh) examined from Thailand are listed in the Supplementary File, and additional selected specimens from Vietnam were also investigated to revise its distribution range (Clemens 3147 [K K001083294!], Clemens 4452 [K K001083319!], Evrard 44 [K K001083320!], Poilane 40706 [K K001083318!]).
Morphologically, P. cordifolia (sensu Roxburgh) differs from P. serratifolia in having cordate or rounded leaf bases (vs. mostly cuneate in P. serratifolia), glabrous vein axils on the lower half of the abaxial leaf surface (vs. densely pubescent), a corolla with a large yellow patch on the inside base of the middle lobe of anterior lip (vs. without a yellow patch), and a longer corolla, 5–7 mm long (vs. 2–5 mm long) (Figs. 2, 5). The study of fruit and endocarp morphology by Satthaphorn et al.50 demonstrated that P. cordifolia produces significantly larger fruits (5.32–6.65 × 4.25–5.66 mm) compared to those of P. serratifolia (3.33–4.89 × 2.58–2.99 mm). The endocarp protrusion of P. cordifolia is type II characterized by sharpened and tapered (thorn-like) protruding structure while P. serratifolia bears type I endocarps with rounded, swollen (saccate-like) protruding structure. Ecological observations and herbarium label data further indicate that P. cordifolia typically occurs in open or shaded primary or secondary evergreen forests, whereas P. serratifolia is usually found in exposed coastal and mangrove habitats (Figs. 5A, 6). Our phylogenetic analyses further support the phylogenetic distinction between P. cordifolia and P. serratifolia, placing them in well-supported separate subclades (Fig. 1). We therefore confirm that P. cordifolia (sensu Roxburgh)57 occurs in Thailand and clearly treated as a distinct species in the Flora of Thailand.
Premna cordifolia can be distinguished from other species regarded in the P. serratifolia complex in Thailand, namely P. paniculata and P. punctulata which are reinstated as distinct species in this study. One key diagnostic feature of P. cordifolia is the presence of interpetiolar ridges on the stem, a character absents in both P. paniculata and P. punctulata. The inflorescence of P. cordifolia is longer (4–8 cm) than that of P. paniculata (0.7–2 cm), though it overlaps in length with P. punctulata. In terms of floral morphology, P. cordifolia exhibits both a yellow patch on the corolla and hairs on the corolla tube, while these features are absent in P. paniculata. Fruit size also provides a distinguishing character, with P. cordifolia bearing larger fruits (5.32–6.65 × 4.25–5.66 mm) compared to P. paniculata (3.22–4.20 × 2.33–3.82 mm). Additionally, the shape of the endocarp protrusions differs: P. cordifolia possesses thorn-like protrusions, whereas P. punctulata exhibits a saccate-like form. Phylogenetic analysis reveals that P. cordifolia occupies a distinct position in subclade I, separate from P. punctulata, which is placed in subclade III. This integrative evidence, encompassing morphological and molecular data, supports the recognition of P. cordifolia (sensu Roxburgh) as a species distinct from both P. paniculata and P. punctulata.
Premna paniculata
H. R. Fletcher, Bull. Misc. Inform. Kew 1938: 201. 1938 & 421. 1938; Leerat. et al., Trop. Nat. Hist. 9(2): 135. 2009 & Thai Forest Bull. (Bot.) 44(2): 122. 2016. Type: Thailand, Prachuap Khiri Khan (Pak Tawan), elev. ca 20 m, 1 August 1931, A.F.G. Kerr 20,536 (lectotype BK BK257574!, selected by Leeratiwong et al.58; isolectotypes BM BM000950181!, E E00284177!, LL LL00375155!, K K001098063!, SING SING0068002!) (Fig. 3).
Premna paniculata. (A, B) habit with fruiting branch; (C) close-up fruits. All photos by C. Leeratiwong.
Description: Scandent shrub to shrub, 2–4 m tall. Branches without an interpetiolar woody ridge, glabrous or brown to reddish-brown pubescent. Leaves decussate, elliptic or ovate-elliptic, 2–11 by (1–)1–5 cm, apex acute or acuminate, base cuneate, margins mostly entire or rarely slightly serrate distally; adaxial surface mostly glabrous, base and margin with sparse brown appressed hairs; abaxial surface glabrous or sometimes pubescent with sparse brown appressed hairs, with or without sparse yellow to brown sessile glands, nerve axils glabrous; petiole 2–5 mm long. Inflorescence corymbose, 0.7–2 cm long; pedicels 0.2–1.5 mm long. Calyx campanulate, 2–2.5 mm long, obscurely and equally 2-lipped; tube 1.5–2 mm long; posterior lip 3-lobed, lobes 0.3–0.8 mm long; anterior lip 2-lobed, lobes 0.4–1 mm long; fruiting calyx 2–3 mm long. Corolla greenish-white or white, 2-lipped, 3–5 mm long; tube 2–3 mm long, glabrous outside, white villous distally inside; posterior lip 1.2–1.5 mm long; anterior lip with middle lobe larger, 1–1.5 mm long, without yellow patch on middle lobe; lobes glabrous on both sides. Stamens long exserted, filaments 1–1.5 mm long; anthers 0.2–0.4 mm long. Ovary glabrous, with or without sparse sessile glands at apex; stylar branches 0.1–0.3 mm long. Fruits green when young, broadly obovoid, 4–6 mm long, glabrous, sparse sessile glands at apex.
Distribution: Endemic to Thailand (Phetchaburi, Prachuap Khiri Khan).
Ecology: In open areas of secondary dry evergreen forests; elevation 20–150 m. Flowering and fruiting June to August.
Vernacular: sak khi kai prachuap (สักขี้ไก่ประจวบ).
Note: Premna paniculata was listed as a synonym of P. serratifolia in the treatment of de Kok3. This inclusion was not explicitly justified, as the species was merely listed under P. serratifolia without further explanation. We presume that de Kok3 considered the type specimens of P. paniculata to represent morphological variation within P. serratifolia. Nevertheless, P. paniculata is geographically restricted to south-western Thailand. Based on comprehensive morphological analyses and ecological evidence, we recognize P. paniculata as a distinct species and reinstate its taxonomic status in this study. This conclusion is further supported by earlier studies8,28,50,58.
The type specimen of P. paniculata (Kerr 20,536 [K K001098063!]) together with three additional specimens (Leeratiwong 16–545 [PSU 01683!], Kerr 20,536 [K K001098063!], Niyomdham 2978 [BKF SN101085!]) can be morphologically distinguished from P. serratifolia by several diagnostic characters. These include the absence of an interpetiolar woody ridge at the stem nodes (vs. presence of an interpetiolar woody ridge in P. serratifolia), glabrous nerve axils on the lower half of the abaxial leaf surface (vs. densely hairy), a glabrous corolla tube externally (vs. distally pubescent), a shorter inflorescence (0.7–2 cm long vs. (0.5–)2–6 cm long), and a posterior calyx lip that is apically 3-lobed (vs. mostly entire to subentire, or rarely 2–3-lobed) (Figs. 3, 5). Endocarp morphology, as documented by Satthaphorn et al.50, further supports the taxonomic separation of these species: P. paniculata exhibits type II endocarps protrusion (thorn-like), whereas P. serratifolia bears type I endocarps protrusion (saccate-like). Additionally, these two species occupy distinct ecological niches, with P. paniculata confined to secondary dry evergreen forests, endemic to Thailand, in contrast to P. serratifolia, which is typically found in coastal or mangrove habitats in wider regions (Fig. 6). Although P. paniculata and P. punctulata are both found in evergreen forest habitats, they can be readily distinguished based on several morphological characters (Table 1). The leaf base of P. paniculata is cuneate, in contrast to the rounded or slightly oblique-rounded base observed in P. punctulata. On the abaxial leaf surface, the nerve axils of P. paniculata are glabrous, whereas those of P. punctulata are sparsely pubescent. Inflorescence length further differentiates the two species, with P. paniculata exhibiting notably shorter inflorescences (0.7–2 cm) compared to the longer ones in P. punctulata (6–20 cm). The posterior calyx lip of P. paniculata is distinctly apically 3-lobed, whereas it is entire to subentire in P. punctulata. Additionally, P. paniculata is also characterized by the absence of a yellow patch on the middle lobe of the anterior corolla lip, but a feature presents in P. punctulata. The endocarp protrusion type also serves as a key diagnostic trait: P. paniculata possesses thorn-like (type II) protrusions, while P. punctulata exhibits saccate-like (Type I) protrusions, as documented by Satthaphorn et al.50. These consistent morphological differences support the recognition of P. paniculata and P. punctulata as distinct species.
We attempted to include P. paniculata in the molecular analyses to clarify its taxonomic placement. However, DNA extraction proved highly challenging due to the limited availability of suitable material. Specimens available for study were mature leaves, and DNA isolated using both CTAB and a commercial extraction kit yielded poor-quality products that consistently failed to amplify. This difficulty is likely attributable to the high levels of polyphenols, polysaccharides, and other secondary metabolites typically accumulated in mature leaf tissues, which are known to interfere with DNA purity and inhibit enzymatic reactions59,60,61,62. Consequently, future studies should prioritize the use of young leaf material for P. paniculata to improve DNA quality and ensure successful molecular analyses.
To clarify the taxonomic status of P. paniculata, the combined evidence from morphological characteristics and ecological information as outlined above provides sufficient resolution to support the recognition as a distinct species from both P. punctulata and P. serratifolia. Accordingly, P. paniculata, which was previously treated as a synonym of P. serratifolia, is here reinstated.
Premna punctulata
C. B. Clarke in Hook.f., Fl. Brit. India 4: 575. 1885; King & Gamble, J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 74: 817. 1909; H. J. Lam, Verbenaceae Malayan Archipel.: 121. 1919; Ridl., Fl. Malay Penins. 2: 620. 1923; Kochummen in Ng, Tree Fl. Malaya 3: 307. 1978; Keng, Con. Fl. Singapore: 194. 1990; Leerat. et al., Trop. Nat. Hist. 8(1): 9. 2008 & 9(2): 135. 2009.—Premna serratifolia sensu de Kok, Fl. Malesiana 23. 327. 2019, pro parte. Type: Malaysia, Malacca, 6 June 1865, Maingay Kew Distribution 1200 (holotype K K000645986!; isotype NY NY00137951!) (Fig. 4).
Premna punctulata. (A) habit with flowering branch; (B) inflorescence; (C) closed-up inflorescence; (D) fruit. Photos A by J. Satthaphorn and B–D by C. Leeratiwong.
Description: Woody climber or rarely scandent shrub. Branches without an interpetiolar woody ridge, glabrous or brown to reddish-brown pubescent. Leaves decussate, ovate to broadly elliptic, 9–15 by 5–9 cm, apex acuminate, acute or rarely retuse, base rounded or slightly oblique-rounded, margins entire; adaxial surface glabrous, midrib sparsely pubescent; abaxial surface brown to reddish-brown pubescent, nerve axils sparsely pubescent, with brown sessile glands; petiole 3–6 cm long. Inflorescence corymbose, 6–20 cm long; pedicels 0.5–1.5 mm long. Calyx pale green to green, 1.5–2 mm long, equally and obscurely 2-lipped; tube 1.5–2 mm long; posterior lip entire to subentire (rarely 2–3-lobed); anterior lip 2-lobed, lobes 0.1–0.15 mm long; fruiting calyx 1–2.5 mm long. Corolla greenish-white or white, 2-lipped, 5–6 mm long; tube 2.7–3.5 mm long, glabrous or sparsely pubescent hairs distally outside, with densely white villous distally inside; posterior lip 1.5–1.7 mm long; anterior lip with middle lobe larger, 1.8–2 mm long, with yellow patch on middle lobe; lobes glabrous on both sides. Stamens long exserted, filaments 2–3 mm long; anthers 0.4–0.5 mm long. Ovary sparsely pubescent and with sessile glands at apex; stigma branches 0.15–0.3 mm long. Fruits black, broadly obovate, 3–7 mm long, glabrous, with or without sessile glands.
Distribution: Thailand (Phatthalung, Songkhla, Narathiwat), Malaysia.
Ecology: In primary to secondary evergreen or dry evergreen forests, along the waterfall; elevation up to 150 m. Flowering March to May, fruiting August.
Vernacular: ย่านใบหอม (yan bai hom) (Songkhla).
Note: Premna punctulata was treated as a synonym of P. serratifolia by de Kok3. However, there was no discussion or justification for this synonymization in the previous study. We infer that this decision was likely based on morphological similarities, as the type specimen of P. punctulata (Maingay 1200 [K K000645986!]) had been subsequently determined as P. serratifolia. Although the phylogenetic analyses presented here placed P. punctulata and P. serratifolia in an unresolved clade in both BI and ML trees (Fig. 1), the relatively long branch length separating P. punctulata suggests considerable genetic divergence53. This molecular distinction aligns with the consistent morphological and ecological difference observed between these two taxa.
Premna punctulata is morphologically distinguished from P. serratifolia by the absence of an interpetiolar woody ridge, a longer corolla, the presence of yellow patches on the middle lobe of the anterior lip of the corolla, and a hairy ovary, whereas P. serratifolia possesses an interpetiolar woody ridge, has a shorter corolla, lacks yellow corolla patches, and has a glabrous ovary (Figs. 4, 5). This taxonomic uncertainty was previously addressed by Satthaphorn et al.50, and demonstrated that the fruit width of P. punctulata is clearly distinct from that of P. serratifolia (3.79–5.69 mm wide vs 2.58–2.99 mm wide, respectively). Furthermore, the two taxa occupy different habitats: P. punctulata occurs in primary to secondary evergreen or dry evergreen forests, whereas P. serratifolia is restricted to coastal and mangrove environments.
Premna serratifolia. (A) habit and habitat; (B) habit; (C) branches with leaves and fruits; (D) inflorescence; (E) closed-up inflorescence; (F) young fruits; (G) mature fruits. All photos by J. Satthaphorn.
The separation of these two taxa is further supported by recent phylogenetic studies conducted by Li et al.5 and Hai et al.17, as well as morphological evidence provided by Leeratiwong et al.8,30 and Satthaphorn et al.50. Based on the combination of consistent morphological distinctions, ecological differentiation, and the relatively long branch length separating P. punctulata from P. serratifolia in our phylogenetic analyses, P. punctulata is here reinstated as a distinct species.
Premna serratifolia L.
Mant. Pl. 2: 253. 1771; Roxb., Fl. Ind. 3: 77. 1832; Schauer in DC., Prodr. 11: 632. 1847; Kurz, Forest Fl. Burma 2: 262. 1877; Munir, J. Adelaide Bot. Gard. 7: 13. 1984; S.L. Chen & M. G. Gilbert in C. Y. Wu & P. H. Raven, Fl. China 17: 26. 1994; A. Rajendran & P. Daniel, Indian Verbenac.: 284. 2002; Leerat. et al., Nat. Hist. J. Chulalongkorn Univ. 9(2): 138. 2009; de Kok, Kew Bull. 68(1): 17. 2013; de Kok in Bramley, Bramley, Fl. Malesiana 23: 327. 2019.—Premna obtusifolia var. serratifolia (L.) Moldenke, Phytologia 28(4): 403. 1974.—Premna obtusifolia f. serratifolia (L.) Moldenke, Phytologia 36(5): 438. 1977; Moldenke & Moldenke in Dassanayake & Fosberg, Rev. Handb. Fl. Ceyl. 4: 342. 1983.Type: India, Hermann Herb. Linn. 782.4 (holotype LINN!) (Fig. 5).
Cornutia corymbosa Burm.f., Fl. Ind.: 132, t. 411, f. 1. 1768, nom. illeg. (non Lam).—Premna integrifolia L., Mant. Pl. Altera: 252. 1771, nom. superfl.—Premna corymbosa (Burm.f.) Schauer in DC., Prodr. 11: 632. 1847, nom. illeg. (non Rottler & Willd.).—Gumira integrifolia Kuntze, Revis. Gen. Pl. 2: 507. 1891, nom. superfl. Type: Sri Lanka [Ceylon], Hermann 2:1 (lectotype BM-HERM BM000621491!, designated by Verdcourt63).
Gumira domestica Hassk., Flora 25, 2, Beibl.: 26. 1842.—Gumira integrifolia Hassk., Cat. Hort. Bot. Bogor.: 135. 1844, nom. superfl. Type: Rumphius, Herb. Amboin. 3: 209, t 134. 1743.
Premna obtusifolia R. Br., Prodr. Fl. Nov. Holland.: 512. 1810.—Premna integrifolia L. var. obtusifolia (R.Br.) C. Pei, Mem. Sci. Soc. China 1: 75. 1932.—Premna corymbosa (Burm.f.) Schauer var. obtusifolia (R.Br.) H. R. Fletcher, Notes Roy. Bot. Gard. Edinburgh 19: 178. 1936. Type: Australia, Prince of Wales Island, Brown s.n. (= Bennett 2324) (holotype BM BM000019556!).
Premna spinosa Roxb., [Hort. Beng.: 46. 1814, nom. nud.] Fl. Ind. 3: 77. 1832; Walp., Report. Bot. Syst. 4: 93. 1845. Type: Roxburgh illustration no. 961 (holotype K!).
Premna foetida Reinw. ex Blume, Bijdr. 14: 816. 1826.—Gumira foetida (Reinw. ex Blume) Hassk., Cat. Hort. Bot. Bogor. 1844; H. J. Lam, Verbenaceae Malayan Archipel.: 153. 1919. Syntypes: Indonesia, Java Reinwardt s.n. (L L0699518!, L0699519!).
Premna gaudichaudii Schauer in DC., Prodr. 11: 631. 1847.—P. obtusifolia var. gaudichaudii (Schauer) Moldenke, Phytologia 27: 69. 1973. Type: Archipelago, Mariannae, 1830, Gaudichuad s.n. (holotype G-DC, n.v.).
Premna sambucina Wall. [Cat. No. 1775, nom. nud.] ex Schauer in DC., Prodr. 11: 631. 1847; Kurz, For. Fl. Burma 2: 261. 1877.—P. corymbosa var. sambucina (Wall. ex Schauer) Moldenke, Known Geogr. Distr. Verbenac. Avicenn.: 68. 1942. Type: India, Moalmyn, 1827, Wallich, Cat. No. 1775 (holotype G-DC, n.v.; isotype K K001114145!).
Premna integrifolia L. var. angustior C. B. Clarke in Hook.f., Fl. Brit. India 4: 574. 1885.—Premna angustior (C. B. Clarke) Ridl., Fl. Malay Penins. 2: 619. 1923.—Premna corymbosa (Burm. f.) Schauer var. angustior (C. B. Clarke) H. R. Fletcher, Notes Roy. Bot. Gard. Edinburgh 19: 178. 1936.—Premna obtusifolia R.Br. var. angustior (C. B. Clarke) Moldenke, Phytologia 5: 87. 1954. Syntypes: Malaysia, Malacca, Griffith 6030 (K K000645926!, K000645927!).
Premna integrifolia L. subsp. truncatolabium H. J. Lam, Verbenaceae Malayan Archipel.: 142. 1919. Type: Papua New Guinea, Kaiser Wilhelmsland, Sepik, Lederman 6572 (lectotype K K000670846!, designated by De Kok3).
Premna integrifolia L. var. minor Ridl., Fl. Malay Penins. 2: 619. 1923.—Premna obtusifolia R. Br. var. minor (Ridl.) Moldenke, Phytologia 5: 88. 1954.—Premna corymbosa (Burm. f.) Schauer var. minor (Ridl.) H. R. Fletcher, Notes Roy. Bot. Gard. Edinburgh 19: 178. 1936. Type: Malaysia, Pahang, Pekan, Ridley s.n. (holotype SING, n.v.).
Description: Scandent shrub or shrub, 0.2–5 m high. Branches with an interpetiolar woody ridge, glabrous or brown to reddish-brown pubescent. Leaves decussate, elliptic, ovate or obovate, (0.5–)2–15 by (0.3–)1.2–10 cm, apex mostly acute or obtuse, rarely acuminate or retuse, leaf base usually cuneate, rarely slightly cordate or rounded, margins entire or serrate distally; adaxial surface glabrous, margins with sparse brown hairs; abaxial surface glabrous or sparse brown pubescent, nerve axils densely pubescent on lower half, with yellow to brown sessile glands; petiole (0.4–)0.8–5.5 cm long. Inflorescence corymbose, 1.7–15 cm long; pedicels 0.2–1.5 mm long. Calyx green, (1–)1.5–2.5 mm long, unequally 2-lipped; tube 1–1.8 mm long; posterior lip mostly entire to subentire or rarely 2–3-lobed, lobes 0.05–0.1 mm; anterior lip 2-lobed, lobes 0.3–1 mm long; fruiting calyx 2–3 mm long. Corolla creamy-white or white, 2-lipped, (2–)3.5–5 mm long; tube (1.8–)2.5–3 mm long, pubescent or sparsely so, with yellow sessile glands at apex outside, densely white villous distally inside; posterior lip 1.2–1.6 mm long; anterior lip with lobes subequal; middle lobe 1.2–2 mm long, without yellow patch on middle lobe; lobes glabrous or sparsely pubescent, with sparse sessile glands outside, glabrous inside. Stamens long exserted, filaments 1–3 mm long; anthers 0.3–0.6 mm long. Ovary 0.5–1 mm long, glabrous, with or without sessile glands; stigma branches 0.05–0.15 mm long. Fruits black, broadly obovoid, 3–8 mm long, glabrous.
Distribution: Thailand (Krungthep Mahanakhon (Bangkok), Samut Sakhon, Chon buri, Rayong, Chantaburi, Trat, Chumphon, Ranong, Suratthani, Phangnga, Phuket, Krabi, Trang, Satun, Songkhla, Narathiwat). Widely distributed throughout the coasts and the islands of tropical and subtropical Asia, Africa, Australia and the Pacific.
Ecology: In open, sandy soil or wetland areas of beach forests and mangrove forests, or strand vegetation along the coastal areas and islands, in tidal zone; elevation from sea level up to 80 m. Flowering and fruiting all year round.
Vernacular: Khet nam man ((เค็ดน้ำมัน), cha lueat (ช้าเลือด) (Trat), man kai (มันไก่), sam pra nga (สามประงา)), sam pra nga bai (สามประงาใบ), akkhi thawan thale (อัคคีทวารทะเล).
Note: Premna serratifolia is a widely distributed species across Southeast Asia. In recent taxonomic revisions in specific regions, numerous synonyms have been associated with this species, particularly from type specimens originating in the Malay Archipelago3. In our study, we focus on defining the species circumscription of P. serratifolia in Thailand, providing a comprehensive morphological description while excluding the concepts of the reinstated species P. paniculata and P. punctulata. The distinguishing morphological features are summarized in Table 1.
Ecologically, P. serratifolia typically inhabits beach forests or coastal habitats, clearly differing from the moist evergreen forest habitats associated with the previously synonymized species (Figs. 5, 6). Field observations indicate that the leaves of P. serratifolia are notably thick leaves and cuticle, likely as an adaptation to protect against salt spray and strong wind from the sea64,65,66. This species may be actively adapted its seed germination facilitated by monsoon climate, mobile substrates, and salinized64,67, while other species may require different mode of seed dispersal and germination. In addition, phylogenetic analysis places three accessions of P. serratifolia, sampled from the opposite sides of peninsular Thailand, within subclade III of Clade II (Fig. 1) which provide the information of phylogenetic placement with its related species.
Distribution map of P. cordifolia, P. paniculata, P. punctulata, and P. serratifolia in Thailand. Numbers near locations refer to the collections cited in Supplementary File.
Although the species circumscription of P. serratifolia in Thailand and its morphologically related species have been clarified in this study, further investigation across a broader geographic range is still required to assess the extent of morphological variation and confirm species delimitation at a monographic scale.
Taxonomic note on Premna octonervia in Thailand
Premna octonervia was listed as a distinct species in the synopsis for Thailand by Leeratiwong et al.8 based on multiple collections from peninsular Thailand, including Kerr 13309 (K K001084832!), Puudjaa et al. 251 (K K00108481!), Chana et al. 251 (BKF SN064958!, KKU 15247!), Leeratiwong 04–53 (BKF SN232004!, KKU 15246!, PSU 0013431!), and Leeratiwong 05–251 (BKF SN232005!, PSU 0013430!). Our re-investigation showed that these specimens is consistent with the original description of P. mollissima rather than P. octonervia. Meanwhile, the actual identity of P. octonervia can be attributed to P. serratifolia, as previously suggested by de Kok3. The dried specimens exhibit an ebony color and a lower density of hairs on the leaf surfaces, slightly distinguishing from those found in other regions, where dried specimens are typically yellowish-brown to yellowish-black and have moderately to densely hairy leaf surfaces. This difference may be attributed to environmental influences affecting the secondary compounds within southern Thailand population.
Taxonomic note on Premna coriacea in Thailand
The specimens identified as P. coriacea (non C. B. Clarke) and P. coriacea var. villosa (non Rajendran & Daniel) were included to Thai account by Leeratiwong et al.8. Our morphological revision supports these two taxa are P. scandens. The actual taxa of P. coriacea (lectotype Law s.n., K K000884638!) and P. coriacea var. villosa (lectotype s.coll. 2912; selected here, K K000884633!) are restricted to the Indian subcontinent and do not occur in Thailand.
In the protologue of P. coriacea var. villosa (C. B. Clarke) A. Rajendran & P. Daniel [= P. villosa C. B. Clarke in Flora of British India by Hooker68], two specimens were cited in the original publication without the designation of the holotype. These two specimens (s.coll. 2912, [K K000884633!] and Beddome s.n. [K K000884632!]) could be considered as syntypes. We therefore selected the specimen of s.coll. 2912 [K000884633] to be the lectotype of the synonymized taxon due to the specimen being cited in the protologue as well as having complete vegetative and reproductive parts.
Taxonomic note on Premna interrupta in Thailand
Premna interrupta Wall. ex Schauer, in A. P. de Candolle, Prodr. 11: 633. 1847.—Premna interrupta var. smitinandii Moldenke, Phytologia 8: 163. 1962, syn. nov.
Leeratiwong et al.8 recognized P. interrupta var. smitinandii based on the presence of villose hairs on the abaxial leaf surface (vs. glabrous leaf in typical variety). This variety was established based on the single collection of Smitinand & Alsterlund 6783 [holotype LL LL00375145!; isotypes K K001084815!, L L0414245!, L0414256!]. After investigation of various specimens belonging to P. interrupta and the type specimen of P. interrupta var. smitinandii, we found that the density of the indumentum on an abaxial leaf surface of P. interrupta var. interrupta is variable among different populations from absent to densely hairy, and leaf maturity. Therefore, we do not recognize P. interrupta var. smitinandii here.