Treatment for benign thyroid nodules and PTC traditionally includes conventional surgical procedures and radioactive iodine treatment [1, 9,10,11,12,13]. However, these methods can lead to significant complications related to thyroid function, parathyroid function, and nerve function. With the advancement of thermal ablation technology, it has gradually become a feasible and acceptable approach for treating thyroid disorders [14, 15]. Numerous studies have indicated that thermal ablation treatment for PTC not only offers effective outcomes but also demonstrates satisfactory safety profiles [16,17,18,19,20], particularly with minimal impact on thyroid function [21], which is in line with our prior research findings [4].
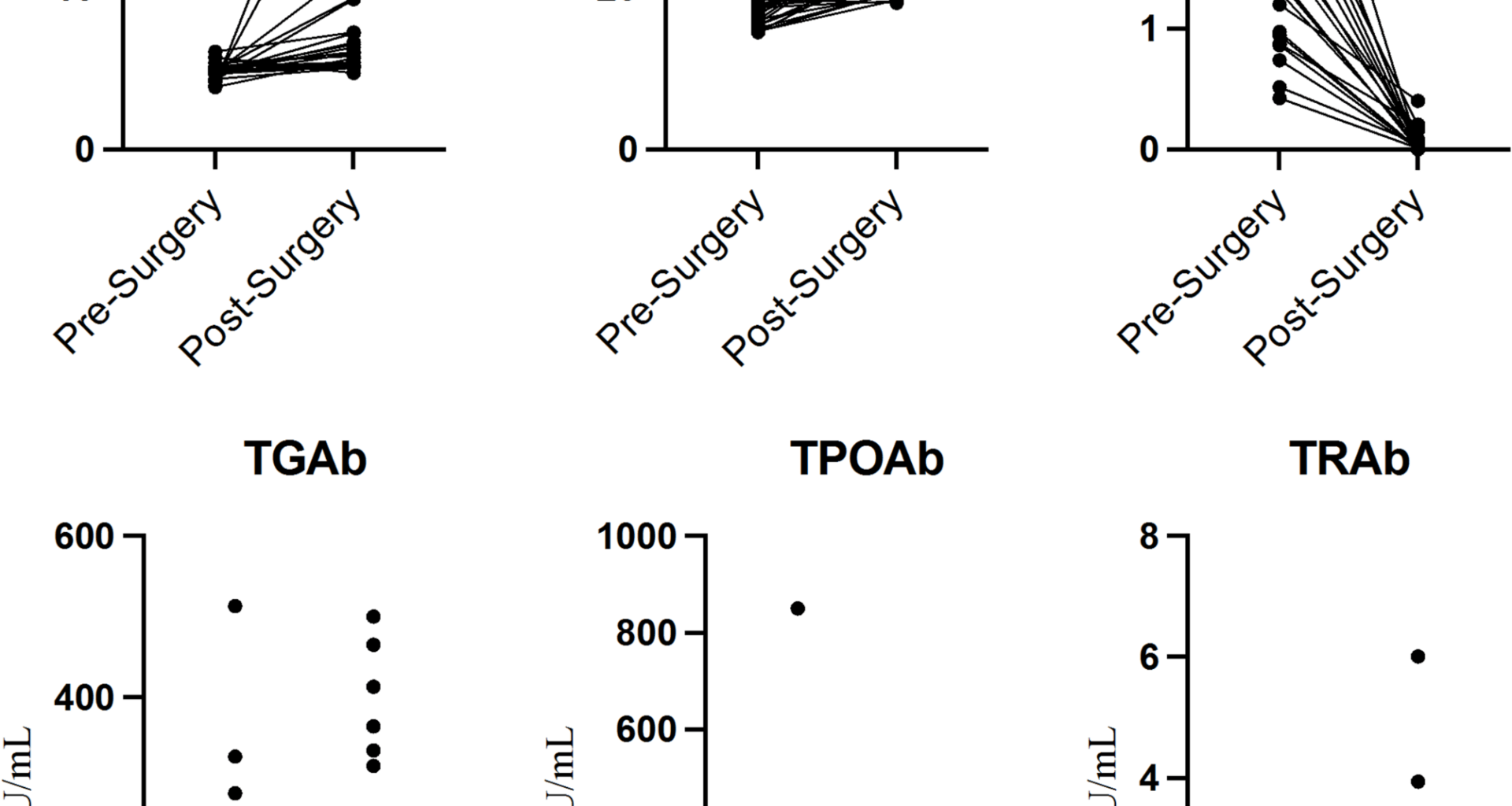
As thermal ablation technology has gained widespread use in thyroid treatment, some complications have begun to be reported and discussed, even though their occurrence rates are lower than those of existing methods. These complications include hoarseness, reactive lymphadenopathy, and radial pain, among others [22, 23]. But with the improvement of technical levels, the above-mentioned deficiencies have gradually declined, which was in line with this research [6, 24]. In this study, we aimed to focus on the occurrence of hyperthyroidism in PTC patients after they underwent thermal ablation. Among the 22 PTC patients, none presented with signs of hyperthyroidism before the procedure. However, during postoperative follow-up, there were instances of decreased TSH levels, along with increased FT3 and/or FT4, accompanied by clinical symptoms of hyperthyroidism, such as heat intolerance, excessive sweating, Hand tremors, and palpitations. Notably, although none of the patients in this study exhibited hyperthyroidism before the procedure, 11 patients had preoperative ultrasound reports that either indicated or raised suspicion of diffuse thyroid lesions, and only 7 patients had no abnormalities associated with thyroid inflammation.
The aetiology of hyperthyroidism is complex, and its primary pathogenic mechanisms remain unclear [25, 26]. However, many studies suggest interactions between genetic and nongenetic factors that ultimately lead to a loss of immune tolerance and the initiation of persistent autoimmune responses against thyroid antigens. Several researchers have reported rare cases of Graves’ disease occurring after thyroid surgery, attributing the disease’s development to changes in the immune status induced by the surgery [27]. A study in Korea [28] reported a 0.2% incidence of Graves’ disease after thyroid cancer surgery, with 61.1% and 44.4% of patients testing positive for TPOAb and TGAb, respectively, through preoperative examination. These findings suggest that preexisting autoimmune thyroid diseases may be a potential factor for the development of Graves’ disease after surgery. Thermal ablation technology induces tissue coagulation and local immune responses via high-frequency energy. On the one hand, the denaturation and necrosis of tumour cells after thermal ablation may expose antigens that enhance the immune system’s ability to attack tumour cells. On the other hand, adjacent normal thyroid tissue inevitably undergoes thermal damage, exposing thyroid antigens, such as thyroglobulin and intracellular antigens [23, 29,30,31,32]. Exposure to these antigens, combined with local blood circulation acceleration, can lead to an immune system attack on healthy thyroid tissue and the release of a significant amount of thyroid hormones. These immune‒inflammatory feedback loops may result in transient subclinical and clinical hyperthyroidism. But we have to stating that, these associations are hypothesis-generating and not intended to guide clinical decision-making without further prospective validation.
Heck et al. [29] studied the effects of thermal ablation on thyroid function and antibodies and reported a significant increase in thyroglobulin levels within 24 h after ablation. When these antigens are taken up by antigen-presenting cells, they induce immune responses via T lymphocytes [23]. A previous study demonstrated that the proportion of T lymphocytes was significantly greater one day and two weeks after thermal ablation, whereas these notable changes no longer appeared one month after thermal ablation. These findings are consistent with our previous research and clinical experience, showing that the majority of patients with thyroid function abnormalities generally recover within a certain period. However, few patients experienced thyroid function disorders during long-term follow-up. We hypothesise that this subgroup may have had subclinical thyroid dysfunction and that thermal ablation treatment acted as a stress factor that accelerated the progression of the disease. Zhao [30] et al. discussed changes in thyroid antibodies following thermal ablation treatment for thyroid nodules. Although a few patients experienced symptoms of hypothyroidism or hyperthyroidism after thermal ablation treatment, none of these patients spontaneously recovered during the follow-up period; furthermore, they suggested that the elevation of TGAb, TPOAb, and TRAb beyond normal ranges after thermal ablation treatment might be related to high preablation levels of TGAb and TPOAb.
Highlights
Although the exact mechanisms underlying the occurrence of hyperthyroidism in PTC patients after thermal ablation treatment are not yet fully understood, several key points can be highlighted: (1) The thermal ablation treatment for 22 patients with PTC proceeded without any complications. There were no postoperative issues, such as hoarseness or coughing during water ingestion. So, the ablation is an alternative treatment option for certain patients with thyroid cancer, particularly for LMICs, elderly patients, or those with comorbidities. (2) PTC patients scheduled for thermal ablation treatment who present with abnormal thyroid-associated antibodies, such as TGAb, TPOAb, or TRAb, in addition to routine thyroid function tests within normal ranges or whose preoperative thyroid ultrasound results suggest the possibility of chronic inflammation should be informed about the potential for thyroid function abnormalities after thermal ablation treatment and the need for close monitoring. (3) For PTC patients who choose thermal ablation as their treatment and subsequently experience hyperthyroidism, regular follow-up and adherence to prescribed medication are essential for timely intervention and management.
Limitations
This study has several limitations. Subgroup analysis indicated no significant difference. However, this might be due to the limit on the number of enrolled groups. The retrospective design and the limited sample size may introduce selection bias, and the absence of long-term follow-up data is another constraint. Therefore, larger-scale prospective multicentre clinical studies are needed to explore long-term thyroid function after thermal ablation treatment and the causes of hyperthyroidism resulting from thermal ablation treatment.