An 18-year-old male patient, a Moroccan international student in China, presented to the emergency department of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, with a chief complaint of persistent upper abdominal pain for one week that had worsened over the past 24 h, accompanied by dyspnea, nausea, and vomiting. On physical examination, he exhibited signs of acute illness, drowsiness, tachypnea (respiratory rate (RR): 40 breaths/min), severe hypoxemia (peripheral oxygen saturation (SpO₂): 80%), hypotension (blood pressure (BP): 80/30 mmHg), tachycardia (heart rate (HR): 120 beats/min), arrhythmia with frequent premature beats, and peripheral cyanosis. Immediate intravenous infusion of vasoactive agents was initiated to stabilize hemodynamics. Transthoracic echocardiography (TTE) showed pulmonary artery dilation, severe pulmonary hypertension, right ventricular (RV) enlargement, diffuse RV hypokinesis, severe tricuspid regurgitation, a high-density intraluminal shadow at the left pulmonary artery origin, and IVC dilation. Lower limb ultrasound demonstrated iliac vein thrombosis. No significant abnormalities were identified on head, chest, and abdominal CT scans. Subsequently, emergency computed tomographic angiography (CTA) of the entire aorta and pulmonary arteries was performed, revealing multiple filling defects involving both main pulmonary arteries and their branches, more prominently on the right side, consistent with acute PE. An urgent consultation with the interventional radiology team was requested immediately.
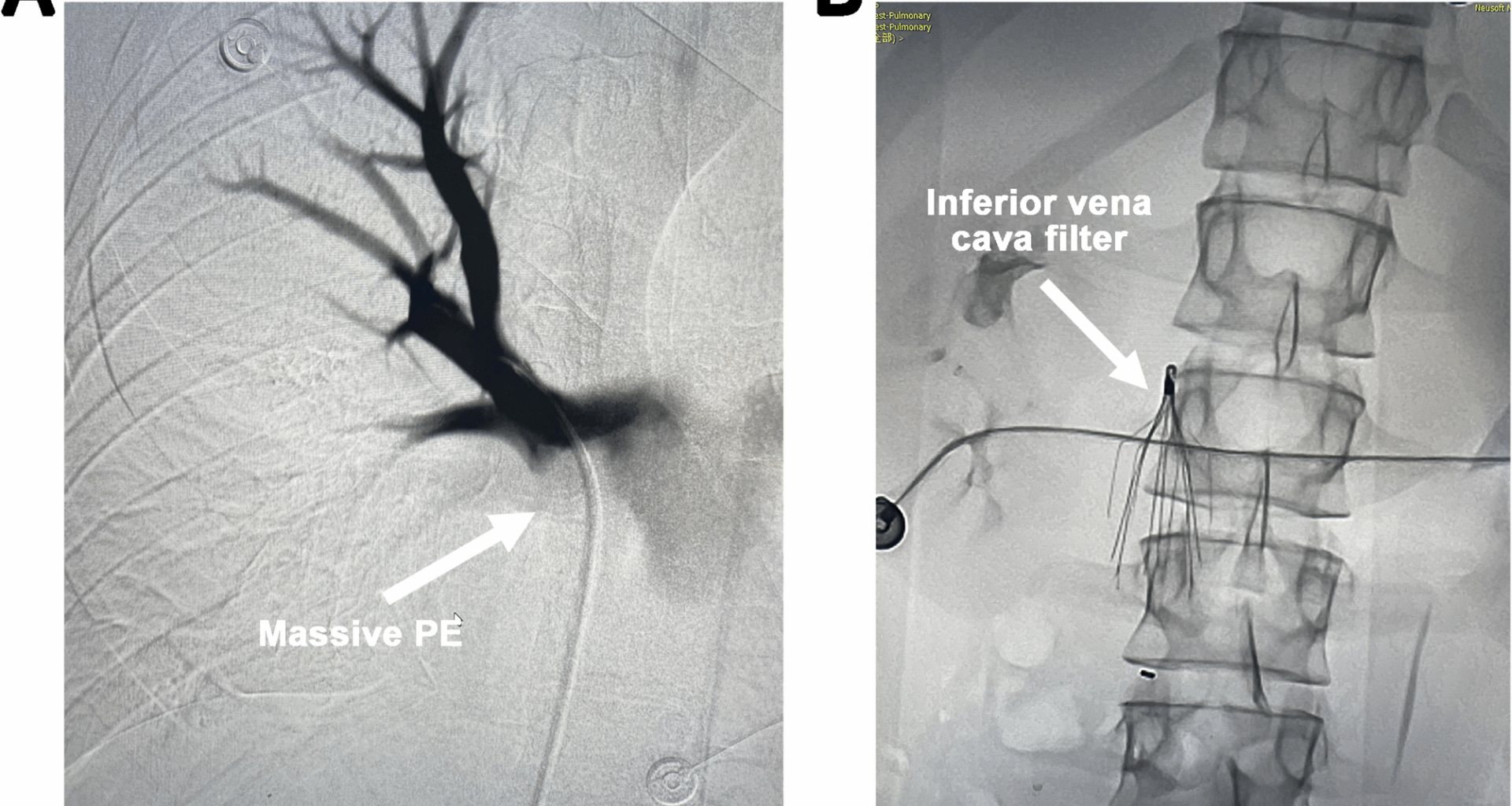
The patient’s condition deteriorated rapidly, with progressive decline in consciousness, SpO2, and BP. His Pulmonary Embolism Severity Index (PESI) score was calculated as 180, corresponding to risk class V, indicating an extremely high mortality risk. Emergency interventions including pulmonary artery thrombectomy, IVC filter placement, iliac vein thrombectomy, pulmonary angiography, and IVC angiography were promptly carried out. Angiographic findings confirmed patent IVC with unobstructed flow; however, scattered filling defects were observed in the main pulmonary arteries and their branches, predominantly on the right side, with significantly impaired blood flow (Fig. 1A). An umbrella-type IVC filter was urgently deployed (Fig. 1B), followed by pulmonary artery thrombectomy. An 8 F guiding catheter was advanced to the right pulmonary artery trunk and branches. Thrombolysis was performed using pulsatile high-pressure injection of 500,000 units of urokinase, followed by mechanical fragmentation with an 8 mm balloon. The Boston Scientific AngioJet 6 F thrombectomy system was then used to aspirate the embolized segment repeatedly, extracting approximately 47 mL of fragmented thrombus. Post-procedural angiography demonstrated a marked reduction in filling defects within the main right pulmonary artery and some of its branches, along with improved blood flow velocity.
(A) Scattered filling defects are observed in the main pulmonary arteries and their branches, predominantly in the right pulmonary artery. (B) Placement of an inferior vena cava (IVC) filter.
Due to the patient’s extremely critical condition, he was urgently transferred to the intensive care unit (ICU) for further life-supporting interventions following the completion of interventional procedures. Upon ICU admission, the patient was in a state of shallow coma with tachypnea. Cardiopulmonary monitoring revealed a HR of 133 beats/min, RR of 27 breaths/min, BP of 131/71 mmHg (maintained with norepinephrine infusion at 0.17 µg/kg/min), and SpO₂ of 68%. Physical examination showed cyanosis of the lips, coarse bilateral lung sounds on auscultation, and arrhythmia. Emergency endotracheal intubation was performed, and invasive mechanical ventilation was initiated using synchronized intermittent mandatory ventilation (SIMV) mode (FiO₂ 1.0, tidal volume 500 mL, RR 24 breaths/min, PEEP 8 cmH₂O). Concurrently, analgesia and sedation were administered, and the norepinephrine dose was increased to 0.3 µg/kg/min to support hemodynamics.
Initial arterial blood gas analysis demonstrated severe acidosis: pH 6.95, PaO₂ 71 mmHg, PaCO₂ 67 mmHg, lactate 9.4 mmol/L, base excess − 18.3 mmol/L. Intravenous administration of 5% sodium bicarbonate was promptly initiated to correct metabolic acidosis, resulting in an improvement of SpO₂ to approximately 90%. However, one hour later, SpO₂ declined gradually to 84%, and BP decreased to 80/60 mmHg despite ongoing norepinephrine infusion at 0.3 µg/kg/min. Repeat arterial blood gas analysis showed: pH 7.23, PaO₂ 50 mmHg, PaCO₂ 62 mmHg, lactate 6.7 mmol/L, HCO₃⁻ 26.0 mmol/L, FiO₂ 1.0. TTE revealed RV dilation with a right ventricle/left ventricle ratio greater than 1 (Supplementary Video 1, Supplementary Video 2). Considering concomitant hematuria, prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT), and the high risk of systemic bleeding associated with thrombolytic therapy, the decision was made—after obtaining informed consent from the patient’s family—to initiate emergency VA-ECMO.
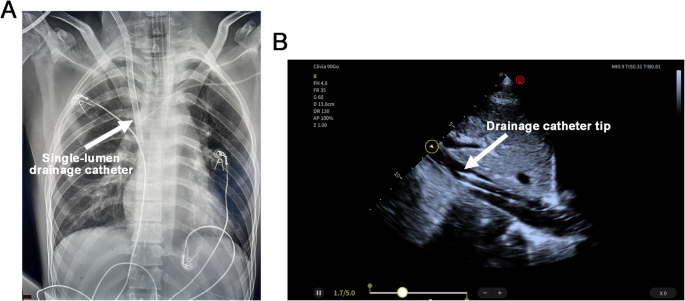
Ultrasound-guided percutaneous cannulation was performed, including placement of a 22-French right IJV drainage catheter and a 19-French femoral artery return catheter. The tip of the drainage catheter was positioned above the hepatic vein confluence within the IVC. Real-time ultrasound guidance was used during insertion, and final catheter positioning was confirmed by chest X-ray and bedside echocardiography (Fig. 2, Supplementary Video 3). The initial ECMO flow was set at 3 L/m²/min. During VA-ECMO support, anticoagulation with unfractionated heparin was administered to maintain APTT between 50 and 60 s, while norepinephrine was continuously infused to maintain mean arterial pressure above 65 mmHg.
The position of the venoarterial extracorporeal membrane oxygenation (VA-ECMO) drainage cannula is confirmed by chest X-ray (A) and transthoracic echocardiography (TTE) (B).
Following initiation of VA-ECMO, the patient’s clinical status gradually improved. Endotracheal extubation was performed on day 3 post-ICU admission, followed by transition to high-flow nasal cannula oxygen therapy. From day 3 to day 5, as cardiac function and hemodynamic parameters stabilized, ECMO support was progressively weaned and ultimately discontinued. On day 7, the patient was switched to low-flow nasal oxygen with stable vital signs. He was transferred to the Department of Respiratory Medicine on day 9 and discharged uneventfully on day 11. Subsequent genetic testing via next-generation sequencing identified a mutation in the F2 gene, confirming the diagnosis of hereditary thrombophilia. Lifelong oral anticoagulation with rivaroxaban was prescribed. Follow-up evaluations indicated that the patient had successfully resumed normal daily activities up to the present time.