McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–60.
Boore JL. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27:1767–80.
Cameron SL. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu Rev Entomol. 2014;59:95–117.
Yuan J, Hu J, Liu W, Chen S, Zhang F, Wang S, et al. Camelus knoblochi genome reveals the complex evolutionary history of Old World camels. Curr Biol. 2024;34:2502–8.
Simon C, Buckley TR, Frati F, Stewart JB, Beckenbach AT. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu Rev Ecol Evol Syst. 2006;37:545–79.
Gan HM, Grandjean F, Jenkins TL, Austin CM. Absence of evidence is not evidence of absence: Nanopore sequencing and complete assembly of the European lobster (Homarus gammarus) mitogenome uncovers the missing nad2 and a new major gene cluster duplication. BMC Genomics. 2019;20:335.
Kieleczawa J. Fundamentals of sequencing of difficult templates—an overview. J Biomol Tech. 2006;17:207–17.
Cameron SL. Insect mitochondrial genomics: a decade of progress. Annu Rev Entomol. 2025;70:83–101.
Treangen TJ, Salzberg SL. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet. 2012;13:36–46.
Formenti G, Rhie A, Balacco J, Haase B, Mountcastle J, Fedrigo O, et al. Complete vertebrate mitogenomes reveal widespread repeats and gene duplications. Genome Biol. 2021;22:120.
Macey JR, Pabinger S, Barbieri CG, Buring ES, Gonzalez VL, Mulcahy DG, et al. Evidence of two deeply divergent co-existing mitochondrial genomes in the Tuatara reveals an extremely complex genomic organization. Commun Biol. 2021;4:116.
Minhas BF, Beck EA, Cheng CHC, Catchen J. Novel mitochondrial genome rearrangements including duplications and extensive heteroplasmy could underlie temperature adaptations in Antarctic notothenioid fishes. Sci Rep. 2023;13:6939.
Jakovlić I, Zou H, Ye T, Zhang H, Liu X, Xiang CY, et al. Mitogenomic evolutionary rates in bilateria are influenced by parasitic lifestyle and locomotory capacity. Nat Commun. 2023;14:6307.
Hardy NB. The biodiversity of Sternorrhyncha: scale insects, aphids, psyllids, and whiteflies. In: Foottit RG, Adler PH, editors. Insect Biodiversity: Science and Society, vol. II. Hoboken: John Wiley & Sons Ltd; 2018. p. 591–625.
García Morales M, Denno BD, Miller DR, Miller GL, Ben-Dov Y, Hardy NB. ScaleNet: a literature-based model of scale insect biology and systematics. Database. 2016;2016:bav118.
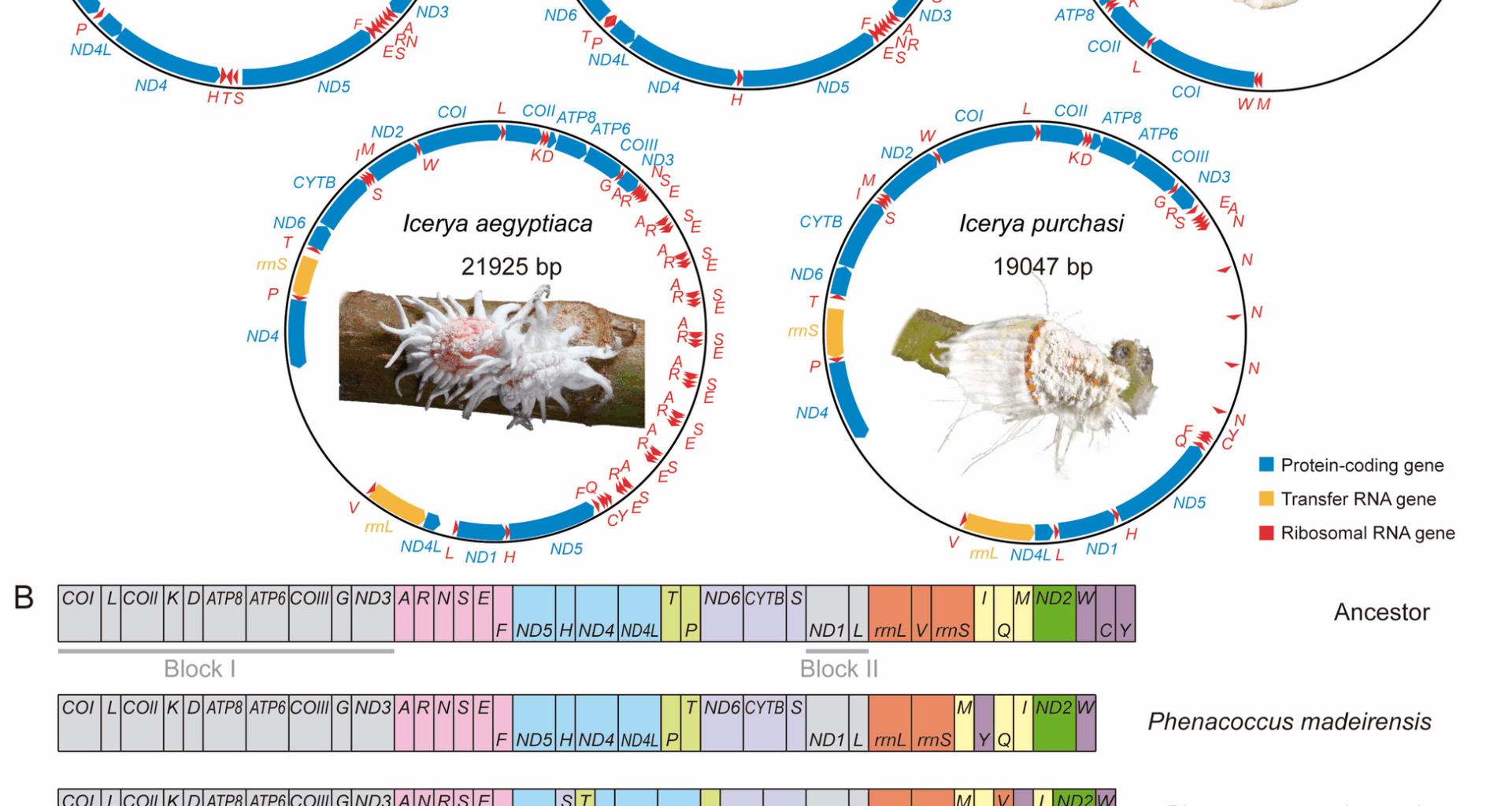
Deng J, Lu C, Huang X. The first mitochondrial genome of scale insects (Hemiptera: Coccoidea). Mitochondrial DNA Part B. 2019;4:2094–5.
Lu C, Huang X, Deng J. Mitochondrial genomes of soft scales (Hemiptera: Coccidae): features, structures and significance. BMC Genomics. 2023;24:37.
Xu H, Liu X, Wang P, Li H, Wu SA. Phylogenetic implications of mitogenomic sequences and gene rearrangements of scale insects (Hemiptera, Coccoidea). Insects. 2023;14:257.
Hu K, Yu S, Zhang N, Tian M, Ban Q, Fan Z, et al. The first complete mitochondrial genome of Matsucoccidae (Hemiptera, Coccoidea) and implications for its phylogenetic position. Biodivers Data J. 2022;10:e94915.
Hodgson CJ, Hardy NB. The phylogeny of the superfamily Coccoidea (Hemiptera: Sternorrhyncha) based on the morphology of extant and extinct macropterous males. Syst Entomol. 2013;38(4):794–804.
Cook LG, Gullan PJ, Trueman HE. A preliminary phylogeny of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea) based on nuclear small-subunit ribosomal DNA. Mol Phylogenet Evol. 2002;25:43–52.
Deng J, Weng X, Ma W, Zhang L, Wang C, Zhou Q, et al. Genomic insights into the phylogeny and evolutionary history of scale insects (Hemiptera: Coccoidea): resolving family-level relationships. Mol Phylogenet Evol. 2025;210:108383.
Camacho ER, Chong JH. General biology and current management approaches of soft scale pests (Hemiptera: Coccidae). J Integr Pest Manag. 2015;6:17.
Herrbach E, Le Maguet J, Hommay G. CHAPTER 11: Virus transmission by mealybugs and soft scales (Hemiptera: Coccoidea). In: Brown JK, editor. Vector-Mediated Transmission of Plant Pathogens. Saint Paul: APS Press; 2016. p. 211–30.
Tong HJ, Ao Y, Li ZH, Wang Y, Jiang MX. Invasion biology of the cotton mealybug, Phenacoccus solenopsis Tinsley: current knowledge and future directions. J Integr Agric. 2019;18:758–70.
Liu Y, Shi J. Predicting the potential global geographical distribution of two Icerya species under climate change. Forests. 2020;11:684.
Shan Y, Gao X, Hu X, Hou Y, Wang F. Current and future potential distribution of the invasive scale Ceroplastes rusci (L., 1758) (Hemiptera: Coccidae) under climate niche. Pest Manag Sci. 2023;79:1184–92.
Reineke A, Karlovsky P, Zebitz CPW. Preparation and purification of DNA from insects for AFLP analysis. Insect Mol Biol. 1998;7:95–9.
Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–90.
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–9.
Dierckxsens N, Mardulyn P, Smits G. NOVOplasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017;45:e18.
Xie Y, Wu G, Tang J, Luo R, Patterson J, Liu S, et al. SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics. 2014;30:1660–6.
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421.
Edgar RC. Muscle5: high-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat Commun. 2022;13:6968.
Uliano-Silva M, Ferreira JGRN, Krasheninnikova K, Darwin Tree of Life Consortium, Formenti G, Abueg L, et al. Mitohifi: a python pipeline for mitochondrial genome assembly from Pacbio high fidelity reads. BMC Bioinformatics. 2023;24:288.
Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–100.
Cheng H, Concepcion GT, Feng X, Zhang H, Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18:170–5.
Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, et al. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 2013;69:313–9.
Chan PP, Lowe TM. tRNAscan-SE: searching for tRNA genes in genomic sequences. Methods Mol Biol. 2019;1962:1–14.
Laslett D, Canbäck B. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 2008;24:172–5.
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022–7.
Perna NT, Kocher TD. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J Mol Evol. 1995;41:353–8.
Zhang Z. KaKs_Calculator 3.0: calculating selective pressure on coding and non-coding sequences. Genom Proteom Bioinf. 2022;20(3):536–40.
Käll L, Krogh A, Sonnhammer E.L.L. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res. 2007;35:W429–32.
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J Mol Biol. 2001;305:567–80.
Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–80.
Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017;33:2583–5.
Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–15.
Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27:1009–10.
Bernt M, Merkle D, Ramsch K, Fritzsch G, Perseke M, Bernhard D, et al. CREx: inferring genomic rearrangements based on common intervals. Bioinformatics. 2007;23:2957–8.
Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 3.81. 2023. http://mesquiteproject.org.
Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–52.
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. Modelfinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–9.
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 2017;34:772–3.
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. Iq-tree 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37:1530–4.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MRBAYES 3.2: efficient Bayesian phylogenetic inference and model selection across a large model space. Syst Biol. 2012;61:539–42.
Butenko A, Lukeš J, Speijer D, Wideman JG. Mitochondrial genomes revisited: why do different lineages retain different genes? BMC Biol. 2024;22:15.
Uliano-Silva M, Ferreira JGRN, Krasheninnikova K, Darwin Tree of Life Consortium, Formenti G, Abueg L, et al. MitoHiFi: a python pipeline for mitochondrial genome assembly from PacBio high fidelity reads. BMC Bioinformatics. 2023;24:288.
Sun X, Wang Y, Chen P, Wang H, Lu L, Ye Z, et al. Biased heteroplasmy within the mitogenomic sequences of Gigantometra gigas revealed by sanger and high-throughput methods. Zool Syst. 2018;43:356–86.
Ladoukakis ED, Zouros E. Evolution and inheritance of animal mitochondrial DNA: rules and exceptions. J Biol Res-Thessaloniki. 2017;24:2.
Morgan B, Wang TY, Chen YZ, Moctezuma V, Burgos O, Le MH, et al. Long-read sequencing data reveals dynamic evolution of mitochondrial genome size and the phylogenetic utility of mitochondrial DNA in Hercules beetles (Dynastes; Scarabaeidae). Genome Biol Evol. 2022;14:evac147.
Novosolov M, Yahalomi D, Chang ES, Fiala I, Cartwright P, Huchon D. The phylogenetic position of the enigmatic, Polypodium hydriforme (Cnidaria, Polypodiozoa): insights from mitochondrial genomes. Genome Biol Evol. 2022;14:evac112.
Kinkar L, Gasser RB, Webster BL, Rollinson D, Littlewood DTJ, Chang BCH, et al. Nanopore sequencing resolves elusive long tandem-repeat regions in mitochondrial genomes. Int J Mol Sci. 2021;22:1811.
Guo ZL, Yuan ML. Research progress of mitochondrial genomes of Hemiptera insects. Sci Sin Vitae. 2016;46:151–66.
Knight RD, Freeland SJ, Landweber LF. A simple model based on mutation and selection explains trends in codon and amino-acid usage and GC composition within and across genomes. Genome Biol. 2001;2:research0010.
Popadin KY, Nikolaev SI, Junier T, Baranova M, Antonarakis SE. Purifying selection in mammalian mitochondrial protein-coding genes is highly effective and congruent with evolution of nuclear genes. Mol Biol Evol. 2013;30:347–55.
Devenish RJ, Papakonstantinou T, Galanis M, Law RH, Linnane AW, Nagley P. Structure/function analysis of yeast mitochondrial ATP synthase subunit 8. Ann N Y Acad Sci. 1992;671:403–14.
Gissi C, Iannelli F, Pesole G. Complete mtDNA of Ciona intestinalis reveals extensive gene rearrangement and the presence of an atp8 and an extra trnM gene in ascidians. J Mol Evol. 2004;58:376–89.
Papakonstantinou T, Galanis M, Nagley P, Devenish RJ. Each of three positively-charged amino acids in the C-terminal region of yeast mitochondrial ATP synthase subunit 8 is required for assembly. Biochim Biophys Acta. 1993;1144:22–32.
Papakonstantinou T, Law RH, Nagley P, Devenish RJ. Non-functional variants of yeast mitochondrial ATP synthase subunit 8 that assemble into the complex. Biochem Mol Biol Int. 1996;39:253–60.
Zhao B, Gao S, Zhao M, Lv H, Song J, Wang H, et al. Mitochondrial genomic analyses provide new insights into the “missing” atp8 and adaptive evolution of Mytilidae. BMC Genomics. 2022;23:738.
Zhang KJ, Zhu WC, Rong X, Zhang YK, Ding XL, Liu J, et al. The complete mitochondrial genomes of two rice planthoppers, Nilaparvata lugens and Laodelphax striatellus: conserved genome rearrangement in Delphacidae and discovery of new characteristics of atp8 and tRNA genes. BMC Genomics. 2013;14(1):417.
Egger B, Bachmann L, Fromm B. Atp8 is in the ground pattern of flatworm mitochondrial genomes. BMC Genomics. 2017;18:414.
Wende S, Platzer EG, Jühling F, Pütz J, Florentz C, Stadler PF, et al. Biological evidence for the world’s smallest tRNAs. Biochimie. 2014;100:151–8.
Pons J, Bover P, Bidegaray-Batista L, Arnedo MA. Arm-less mitochondrial tRNAs conserved for over 30 millions of years in spiders. BMC Genomics. 2019;20:665.
Wolstenholme DR, Macfarlane JL, Okimoto R, Clary DO, Wahleithner JA. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987;84:1324–8.
Xue XF, Guo JF, Dong Y, Hong XY, Shao R. Mitochondrial genome evolution and tRNA truncation in Acariformes mites: new evidence from eriophyoid mites. Sci Rep. 2016;6:18920.
Beckenbach AT, Joy JB. Evolution of the mitochondrial genomes of gall midges (Diptera: Cecidomyiidae): rearrangement and severe truncation of tRNA genes. Genome Biol Evol. 2009;1:278–87.
Zhang J. Recognition of the tRNA structure: everything everywhere but not all at once. Cell Chem Biol. 2024;31:36–52.
Watanabe Y, Suematsu T, Ohtsuki T. Losing the stem-loop structure from metazoan mitochondrial tRNAs and co-evolution of interacting factors. Front Genet. 2014;5:109.
Jühling T, Duchardt-Ferner E, Bonin S, Wöhnert J, Pütz J, Florentz C, et al. Small but large enough: structural properties of armless mitochondrial tRNAs from the nematode Romanomermis culicivorax. Nucleic Acids Res. 2018;46:9170–80.
Hennig O, Philipp S, Bonin S, Rollet K, Kolberg T, Jühling T, et al. Adaptation of the Romanomermis culicivorax CCA-adding enzyme to miniaturized armless tRNA substrates. Int J Mol Sci. 2020;21:9047.
Ohtsuki T, Watanabe Y, Takemoto C, Kawai G, Ueda T, Kita K, et al. An “elongated” translation elongation factor Tu for truncated tRNAs in nematode mitochondria. J Biol Chem. 2001;276:21571–7.
Sato A, Suematsu T, Aihara KK, Kita K, Suzuki T, Watanabe K, et al. Duplication of Drosophila melanogaster mitochondrial EF-Tu: pre-adaptation to T-arm truncation and exclusion of bulky aminoacyl residues. Biochem J. 2017;474:957–69.
Jühling F, Pütz J, Bernt M, Donath A, Middendorf M, Florentz C, et al. Improved systematic tRNA gene annotation allows new insights into the evolution of mitochondrial tRNA structures and into the mechanisms of mitochondrial genome rearrangements. Nucleic Acids Res. 2012;40:2833–45.
Kuhle B, Hirschi M, Doerfel LK, Lander GC, Schimmel P. Structural basis for shape-selective recognition and aminoacylation of a D-armless human mitochondrial tRNA. Nat Commun. 2022;13:5100.
Kuhle B, Hirschi M, Doerfel LK, Lander GC, Schimmel P. Structural basis for a degenerate tRNA identity code and the evolution of bimodal specificity in human mitochondrial tRNA recognition. Nat Commun. 2023;14:4794.
Bhattacharyya SN, Adhya S. The complexity of mitochondrial tRNA import. RNA Biol. 2004;1:84–8.
Masta SE, Boore JL. Parallel evolution of truncated transfer RNA genes in arachnid mitochondrial genomes. Mol Biol Evol. 2008;25:949–59.
Ye F, Li H, Xie Q. Mitochondrial genomes from two specialized subfamilies of Reduviidae (Insecta: Hemiptera) reveal novel gene rearrangements of true bugs. Genes. 2021;12:1134.
Zhang H, Lu C, Liu Q, Zou T, Qiao G, Huang X. Insights into the evolution of aphid mitogenome features from new data and comparative analysis. Animals. 2022;12:1970.
Boore JL, Brown WM. Big trees from little genomes: mitochondrial gene order as a phylogenetic tool. Curr Opin Genet Dev. 1998;8:668–74.
Montaña-Lozano P, Moreno-Carmona M, Ochoa-Capera M, Medina NS, Boore JL, Prada CF. Comparative genomic analysis of vertebrate mitochondrial reveals a differential of rearrangements rate between taxonomic class. Sci Rep. 2022;12:5479.
Boore JL. The duplication/random loss model for gene rearrangement exemplified by mitochondrial genomes of deuterostome animals. In: Sankoff D, Nadeau J, editors. Comparative Genomics. Dordrecht: Kluwer Academic Publishers; 2000. p. 133–47.
Dowton M, Campbell NJ. Intramitochondrial recombination-is it why some mitochondrial genes sleep around? Trends Ecol Evol. 2001;16:269–71.
Lavrov DV, Boore JL, Brown WM. Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: duplication and nonrandom loss. Mol Biol Evol. 2002;19:163–9.
Rawlings TA, Collins TM, Bieler R. Changing identities: tRNA duplication and remolding within animal mitochondrial genomes. Proc Natl Acad Sci U S A. 2003;100:15700–5.
Li M, Schönberg A, Schaefer M, Schroeder R, Nasidze I, Stoneking M. Detecting heteroplasmy from high-throughput sequencing of complete human mitochondrial DNA genomes. Am J Hum Genet. 2010;87:237–49.
Sweet AD, Johnson KP, Cameron SL. Independent evolution of highly variable, fragmented mitogenomes of parasitic lice. Commun Biol. 2022;5:677.
Wu N, Liu J, Wang S, Guo X. Comparative analysis of mitochondrial genomes in two subspecies of the sunwatcher toad-headed agama (Phrynocephalus helioscopus): prevalent intraspecific gene rearrangements in Phrynocephalus. Genes. 2022;13:203.
Xia Y, Zheng Y, Murphy RW, Zeng X. Intraspecific rearrangement of mitochondrial genome suggests the prevalence of the tandem duplication-random loss (TDLR) mechanism in Quasipaa boulengeri. BMC Genomics. 2016;17:965.
Lunt DH, Whipple LE, Hyman BC. Mitochondrial DNA variable number tandem repeats (VNTRs): utility and problems in molecular ecology. Mol Ecol. 1998;7:1441–55.
Levinson G, Gutman GA. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987;4:203–21.
Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435–45.
Ray DA, Densmore LD. Repetitive sequences in the crocodilian mitochondrial control region: poly-A sequences and heteroplasmic tandem repeats. Mol Biol Evol. 2003;20:1006–13.
Verscheure S, Backeljau T, Desmyter S. Dog mitochondrial genome sequencing to enhance dog mtDNA discrimination power in forensic casework. Forensic Sci Int Genet. 2014;12:60–8.
Zhang DX, Hewitt GM. Insect mitochondrial control region: a review of its structure, evolution and usefulness in evolutionary studies. Biochem Syst Ecol. 1997;25:99–120.
Liang G, Mi D, Chang J, On Yau T, Xu G, Ruan J, et al. Precise annotation of Drosophila mitochondrial genomes leads to insights into AT-rich regions. Mitochondrion. 2022;65:145–9.
White MM, Martin HR. Structure and conservation of tandem repeats in the mitochondrial DNA control region of the least brook lamprey (Lampetra aepyptera). J Mol Evol. 2009;68:715–23.
Gupta R, Kanai M, Durham TJ, Tsuo K, McCoy JG, Kotrys AV, et al. Nuclear genetic control of mtDNA copy number and heteroplasmy in humans. Nature. 2023;620:839–48.