Jan BalajkaInstitute of Applied Physics, Vienna University of Technology, Vienna, Austria
November 10, 2025• Physics 18, 180
A scheme combining a scanning probe microscope with a quantum sensor can locally trigger water dissociation and observe the elementary steps of such a reaction.
APS/Carin Cain
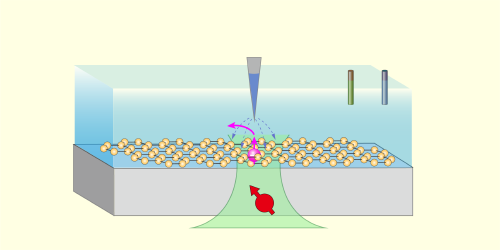
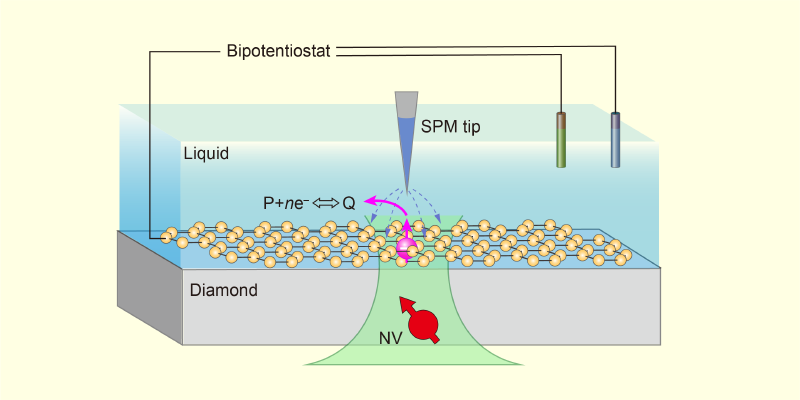
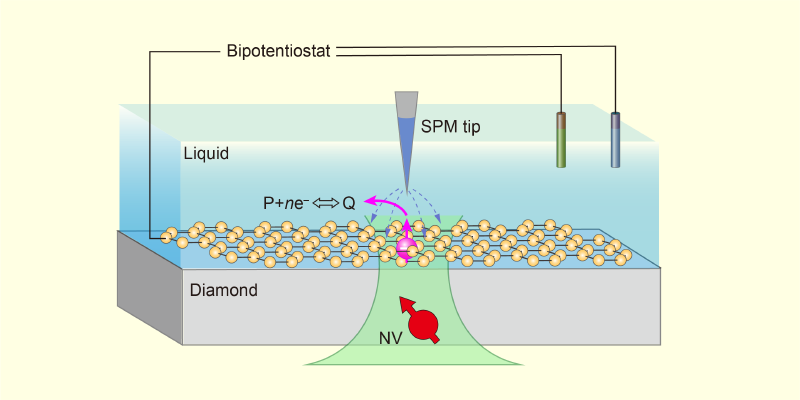
Figure 1: The schematic shows a proposed application of NV-SPM. Within a liquid cell, a 2D material (yellow) can be used as a model interface. Controlled by a bipotentiostat, the scanning probe microscopy tip not only characterizes one of the material’s active sites (pink) but also triggers the interfacial reaction P + ne–⇔Q, where P and Q represent the reactants and products.
APS/Carin Cain
Figure 1: The schematic shows a proposed application of NV-SPM. Within a liquid cell, a 2D material (yellow) can be used as a model interface. Controlled by a bipotentiostat, the scanning probe microscopy tip not only characterizes one of the material’s active sites (pink) but also triggers the interfacial reaction P + ne–⇔Q, where P and Q represent the reactants and products.×
Every experimental technique comes with trade-offs. High-resolution microscopy can pinpoint the positions of individual atoms, yet it typically cannot identify them chemically. Spectroscopy provides chemical information but often only as an averaged signal over a large region. To construct a comprehensive picture of processes at the nanoscale, researchers often resort to combining two or more independent methods. The metaphorical silver bullet would be a single technique that is both local and capable of identifying chemical species as they form and react. Now Wentian Zheng of Peking University and his collaborators have taken an impressive step toward that goal. They have combined two previously separate capabilities—quantum sensing and scanning probe microscopy (SPM)—into a single instrument that can trigger and observe chemical reactions with nanometer resolution [1]. To showcase their new instrument’s power, the researchers monitored the elementary steps of water dissociation at a solid–liquid interface, a reaction of central importance in chemistry, biology, and earth science.
To appreciate this achievement, consider some of the existing techniques for surface and materials analysis. Nonlinear optical spectroscopies can selectively detect molecules at the interface, but their signals are spatially averaged. SPM offers unrivaled spatial resolution for imaging and manipulating surfaces, and derived methods such as tip-enhanced Raman spectroscopy can even distinguish individual molecular isomers [2]. However, these methods probe vibrational modes of chemical bonds and cannot detect unpaired electrons and radicals—important intermediates in chemical reactions. Electron spin resonance (ESR) and nuclear magnetic resonance (NMR) reveal the structure of molecules and their local environment, but they are inherently bulk techniques lacking spatial resolution.
The researchers’ work builds on an emerging nanoscale diagnostic technique that has shown remarkable power: quantum sensing with nitrogen-vacancy (NV) centers in diamond. NV centers are point defects consisting of a nitrogen atom next to a lattice vacancy. These defects exhibit a photoluminescence whose intensity depends on their spin state. That dependence, in turn, responds to external stimuli such as electric and magnetic fields. This sensitivity allows the use of ESR and NMR to probe the NV centers and to monitor physical phenomena in their proximity. For example, a shallow NV center located just beneath the surface can detect and identify chemical species at the interface [3–5]. However, until now, the ability to trigger a reaction at the very location where the NV center is sensing had been missing.
The key component enabling this breakthrough is a scanning quantum sensing microscope called NV-SPM [6], which is based on the qPlus force sensor [7]. This off-the-shelf sensor incorporates a quartz tuning fork to achieve stable operation at room temperature and pressure or in a liquid [8, 9]. In their demonstration, Zheng and his collaborators positioned the microscope’s atomically sharp metallic and electrically biased tip above a shallow subsurface NV center. The strong local electric field extracted photoactivated electrons from the diamond into the thin layer of interfacial water, effectively injecting electrons into the hydration layer and initiating chemical reactions right at the tip.
Once electrons were injected into the interfacial water, hydrated electrons formed. These short-lived species, which are responsible for the emergence of metallicity in water [10] and other exotic phenomena, are powerful reducing agents. In the team’s demonstration, the hydrated electrons triggered a sequence of redox reactions. First, water molecules dissociated into hydroxide ions (OH–) and protons. Then, under the strong electric field of the tip, OH– converted to neutral hydroxyl radicals, releasing additional hydrated electrons. Last, the unstable hydroxyl radicals combined to form hydrogen peroxide, which promptly decomposed into water and oxygen.
Detecting the intermediate species in the redox reactions was only possible because of the two technologies that the researchers combined. The NV center acted as a built-in ESR or NMR sensor, enabling the detection of unpaired electrons in reaction intermediates. In addition, by monitoring the lifetime of proton signals from OH and water within the nanometer-scale detection volume of the NV center, the researchers characterized proton diffusion at the interface.
NV-SPM could be applied beyond the diamond–water interface studied by Zheng and his collaborators. Although a diamond substrate is required to provide NV centers, the surface could be modified with evaporated electrodes—to investigate electrochemical reactions—or covered with various 2D materials—to investigate chemical reactions at different solid–liquid interfaces (Fig. 1). In these systems, electrons injected via the biased SPM tip would also trigger chemical reactions locally, while nanoscale-resolution ESR and NMR could be used to monitor electron transfer, identify reaction intermediates and products, and analyze diffusion dynamics.
Some of the best scientific advances arise from combining ideas that previously existed in isolation. By merging quantum sensing with SPM, Zheng and his collaborators have shown that chemical reactions at solid–liquid interfaces can be probed with both nanoscale resolution and chemical specificity. Although challenges remain, particularly when moving to complex chemical environments, the proof of principle is compelling. With tools like NV-SPM, we may soon be able to uncover the molecular details of interfacial reactions not by inference but by direct observation.
ReferencesW. Zheng et al., “Probing interfacial water dissociation at the nanoscale with a quantum sensor,” Phys. Rev. Lett. 135, 208001 (2025).S. Mahapatra et al., “Tip-enhanced Raman spectroscopy: Chemical analysis with nanoscale to angstrom scale resolution,” J. Chem. Phys. 153, 010902 (2020).T. Staudacher et al., “Nuclear magnetic resonance spectroscopy on a (5-nanometer)3sample volume,” Science 339, 561 (2013).N. Aslam et al., “Nanoscale nuclear magnetic resonance with chemical resolution,” Science 357, 67 (2017).S. J. DeVience et al., “Nanoscale NMR spectroscopy and imaging of multiple nuclear species,” Nature Nanotechnol. 10, 129 (2015).K. Bian et al., “A scanning probe microscope compatible with quantum sensing at ambient conditions,” Rev. Sci. Instrum. 95, 053707 (2024).F. J. Giessibl, “The qPlus sensor, a powerful core for the atomic force microscope,” Rev. Sci. Instrum. 90, 011101 (2019).K. Pürckhauer et al., “Imaging in biologically-relevant environments with AFM using stiff qPlus sensors,” Sci. Rep. 8, 9330 (2018).A. Auer et al., “Electrochemical AFM/STM with a qPlus sensor: A versatile tool to study solid-liquid interfaces,” J. Chem. Phys. 159, 174201 (2023).P. E. Mason et al., “Spectroscopic evidence for a gold-coloured metallic water solution,” Nature 595, 673 (2021).About the Author
Jan Balajka is an assistant professor at the Vienna University of Technology (TU Wien), where he and his group develop techniques to explore materials across a wide range of environments with atomic precision. He earned a PhD in physics in 2018 from TU Wien. Before rejoining TU Wien in 2020, he was a postdoctoral fellow at Cornell University.
Probing Interfacial Water Dissociation at the Nanoscale with a Quantum Sensor
Wentian Zheng, Ke Bian, Jiyu Xu, Xiakun Chen, Shichen Zhang, Rainer Stöhr, Andrej Denisenko, Jörg Wrachtrup, Sheng Meng, and Ying Jiang
Phys. Rev. Lett. 135, 208001 (2025)
Published November 10, 2025
Read PDFSubject AreasChemical PhysicsNanophysicsRelated Articles
 More Articles
More Articles