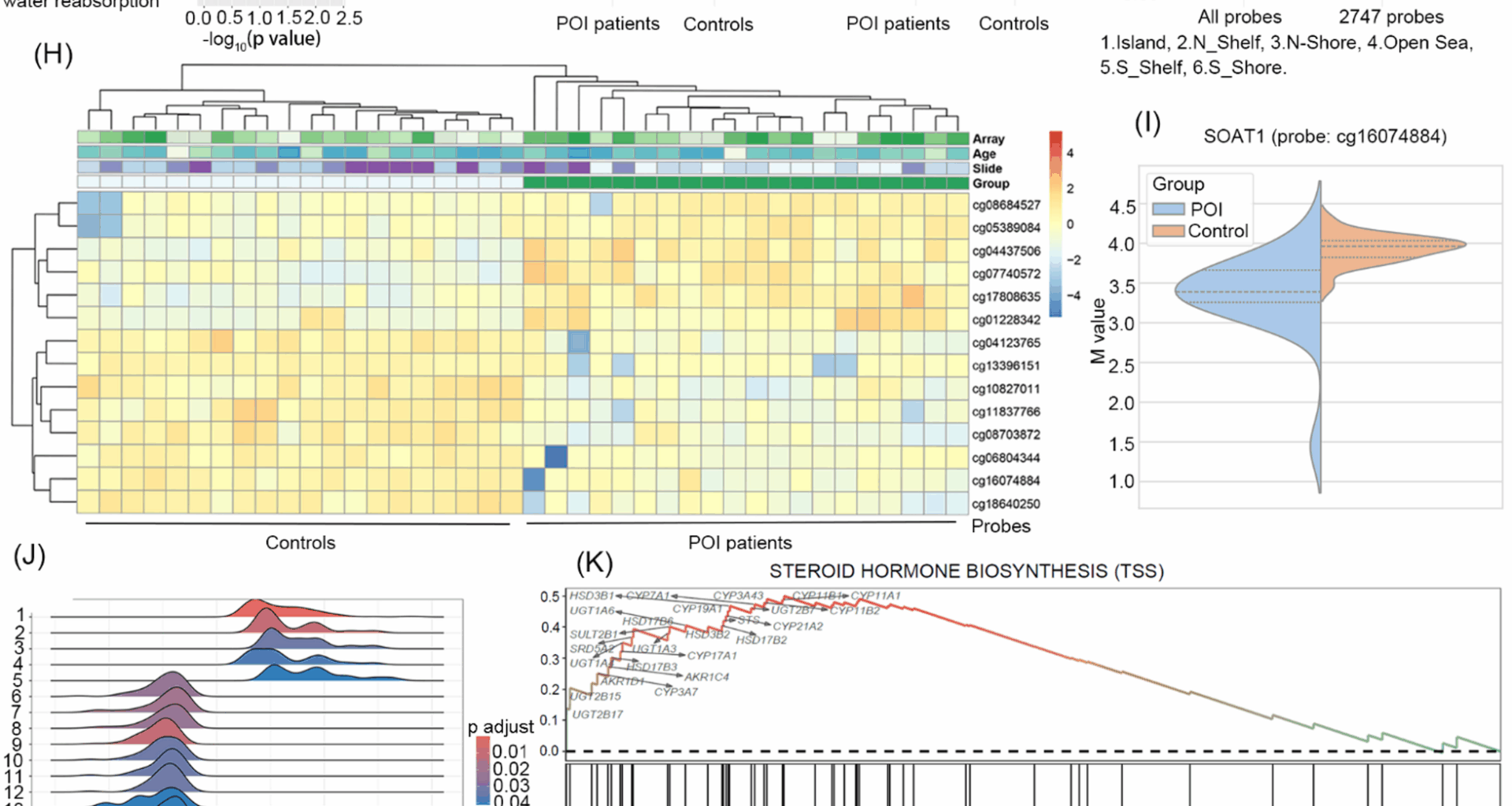
Premature ovarian insufficiency (POI) is associated with an increased risk of neurodegenerative diseases, but the underlying mechanisms remain unclear. Here, we integrated DNA methylome profiling of peripheral blood leukocytes and circulating steroid hormone analysis to identify potential mechanism linking POI to neurogenerative risk. Methylome analysis revealed distinct epigenetic signatures in POI patients, including hypomethylation at the SOAT1 promoter, a gene critical for cholesterol homeostasis. Gene set enrichment analysis (GSEA) implicated suppressed steroid biosynthesis, supported by significantly reduced circulating levels of steroids, including androstenedione, dehydroepiandrosterone (DHEA), aldosterone, cortisol, and cortisone in POI patients. Notably, neuroprotective steroids DHEA and pregnenolone exhibited an age-dependent decline exclusively in the POI group. Our findings suggest that SOAT1-mediated cholesterol dysmetabolism leads to steroidogenesis suppression and depletion of neuroprotective steroids. Epigenetic dysregulation of SOAT1 and steroidogenic genes, coupled with depletion of DHEA and pregnenolone might contribute to the elevated neurodegenerative risk in POI.
Epigenetic dysregulation of steroidogenesis and neuroactive steroid deficiency in premature ovarian insufficiency: implications for neurodegenerative risk | Biomarker Research
- November 13, 2025
