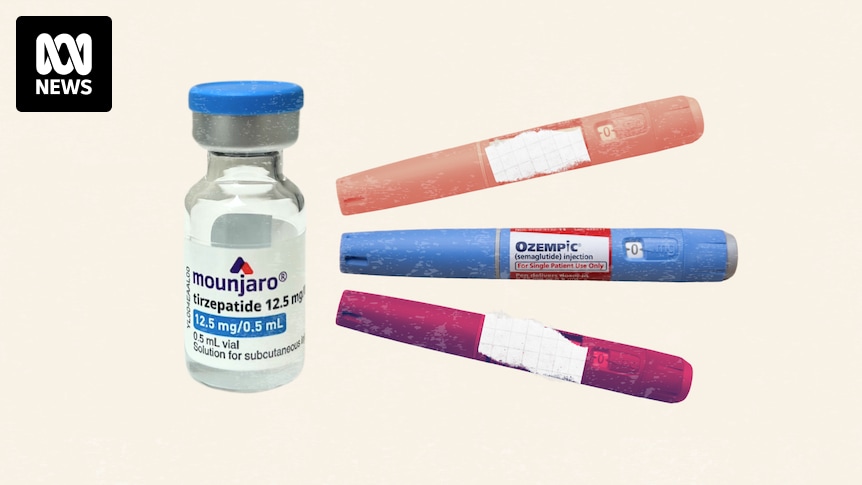
Peak eating disorder bodies are raising concerns about the rapid rollout of GLP-1 weight-loss treatments through telehealth, and what they describe as aggressive social media marketing by some companies.
In one case, a company referred to “pesky Aussie laws” as having prevented it from providing more information about the medication to potential customers.
The Australia and New Zealand Academy for Eating Disorders said it was concerned such marketing techniques could target people who might not need the medication.
“Very, very little medical monitoring, or medical history [is] being taken [by telehealth],” chief executive officer Jade Gooding said.Loading…
Brisbane-based Ms Gooding said eating disorder groups were aware of people misusing the medication and accessing it when they should not have.
“We hold concerns about how these particular medications are being prescribed by [telehealth providers].”
International company Juniper is among their concerns, having run a recent Black Friday sale.
Juniper is part of EUC Management, also known as Eucalyptus, which has operations in Australia, the UK, Germany and Japan.
Its revenue more than doubled to $248 million last financial year, according to accounts filed for 2025 with regulators.
It still lost $25 million last year, and its biggest cost was marketing at $86.1 million, almost tripling from the year before.
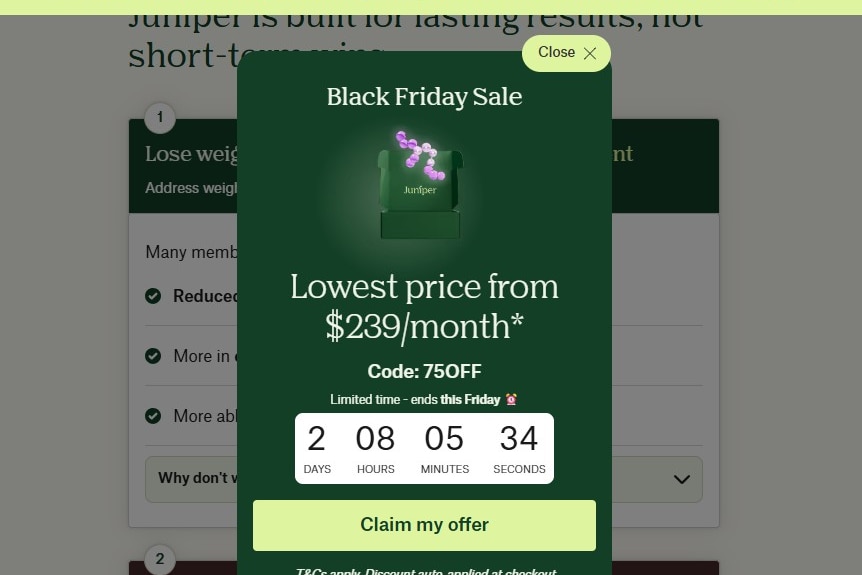
Juniper is a telehealth company that ran a Black Friday sale in November. (Supplied)
A company that operates under the same umbrella, Pilot, sells similar medical weight-loss treatments but targets a male audience, while Juniper targets women.
Practitioners from both companies can prescribe Ozempic-style drugs, including Wegovy (semaglutide) and Mounjaro (tirzepatide).
Black Friday sales and ‘pesky Aussie laws’
The ABC asked Juniper about a past Black Friday sale and how it related to prescription medication, after advertisements promoting the sale included discount codes and marketing emails.
Eucalyptus clinical director Matt Vickers said in a statement, “any promotional activity relates solely to the cost of accessing our medical service, not the cost of a prescription medication”.
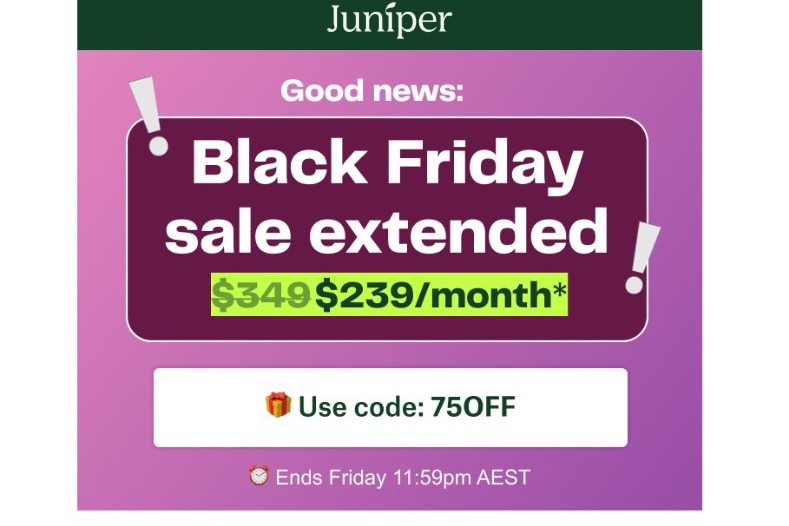
Juniper sent various “Black Friday” sale content to its patients. (Supplied)
Brisbane student Evangeline Gardiner was among those who said they had been “bombarded” with advertisements.
“It does make me feel like they only care about sales; they don’t care about me or my health as an individual person,” she said.
The 30-year-old, who had previously experienced disordered eating, said she found the advertisements particularly triggering.
“You’d never see a Black Friday sale for a pap smear or a cancer screening or skin check,” she said.
“It really doesn’t sit right for me. You could have a Black Friday sale for something like that.”
Loading…
The ABC sighted comments on Juniper’s Facebook page, which included one response that told a user the company would direct message them with further information because of “pesky Aussie laws”.
In Australia, strict laws prohibit the public advertising of prescription medicines.
The ABC does not suggest that Eucalyptus is breaching the law.
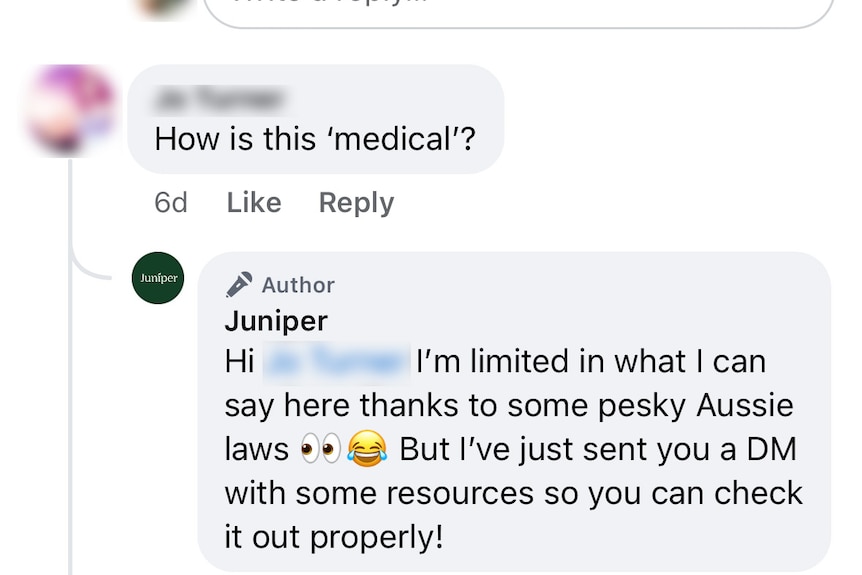
Juniper says “we regret the remark and acknowledge that it may have caused concern”. (Facebook)
The advertising restrictions do not capture promotion of a health service that does not promote any therapeutic good and only refers to the type of consultation the service offers.
Juniper said the comment the company made on social media did not reflect the organisation’s view or approach to regulations.
The company frequently replied to social media users, asking them to message privately for more information about the medical weight loss treatment.
“We take compliance seriously and fully support Australia’s regulatory framework governing telehealth and prescription medicines,” Dr Vickers said in a statement.
Eating disorder resources:
“We regret the remark and acknowledge that it may have caused concern.
“The comment has been removed, and we have reviewed our internal processes to inform improvements.
“We remain committed to high standards of clinical governance and regulatory compliance.”
Dr Vickers said the previous Black Friday sale “did not influence a clinician’s decision to prescribe; eligibility is always determined through a structured clinical assessment”.
“We recognise that promotional activity in this space must be handled carefully, which is why our clinical safeguards and screening processes remain unchanged and consistently applied to every patient,” he said.
Matt Vickers says “any promotional activity relates solely to the cost of accessing our medical service”. (Supplied)
The ABC also sighted logos for organisations, including the Royal Australian College of General Practitioners (RACGP) and the Australian College of Nurse Practitioners, on Juniper’s website.
After the ABC raised questions, the logos were removed.
In a statement, a spokesperson for the RACGP said “weight loss medications should only be prescribed within clear clinical criteria and ongoing monitoring frameworks”.

RACGP, ACHS and ACNP logos (above) were removed from Juniper’s website following ABC questioning (below). (Supplied)
The ABC understands the RACGP does not provide endorsement to Juniper.
Dr Vickers said “we previously displayed the Royal Australian College of General Practitioners (RACGP) logo to acknowledge that our prescribing workforce includes Fellows of the College”.
Markets feel the Ozempic effect
“To avoid any misunderstanding or perception that the college formally endorses our operations, the logo has now been removed,” he said in a statement.
The Australian Council on Healthcare Standards logo was also removed after questioning.
The council said it accredited Eucalyptus.
Stronger safeguards needed
Executive director of Eating Disorders Families Australia Jane Rowan said stronger safeguards were needed to access medical weight-loss treatments.
Australia’s medicines regulator recently issued a safety warning over the potential risk of suicidal thoughts and behaviours when taking these drugs.
She said there also needed to be “more rigour” around the advertising of medical weight-loss treatments and that, in her view, the federal government needed to be involved.
“I think when these companies are advertising as widely as they are without considerations of the vulnerable people out there — those who may be at risk of developing an eating disorder — that’s a real problem, and we certainly need regulations to address.”
Ms Rowan, who has type 1 diabetes, was prescribed Ozempic by her endocrinologist in September 2023 as part of her diabetes management.
She said her work in the eating disorder space meant she understood both the need for the medication and the importance of regulation.
“There’s certainly a place for these medications, but they do need to be done under close supervision and for appropriate medical reasons.”

Jane Rowan was prescribed Ozempic by her endocrinologist as part of her diabetes management. (ABC News: Shari Hams)
The Therapeutic Goods Administration (TGA) said the government was not able to comment on whether the companies mentioned were engaging in unlawful advertising of therapeutic goods.
The ABC also contacted federal Health Minister Mark Butler, who responded in conjunction with the TGA.
“Under the Therapeutic Goods Act 1989 [the act], it is an offence to advertise a therapeutic good where the advertisement ‘refers to’ a prescription-only medicine,” a TGA spokesperson said in a statement.
None of the companies’ advertising mentioned any prescription-only medicine.
Loading…
The spokesperson said the TGA strongly urged Australians using or considering weight-loss products — especially from overseas or online — to check safety alerts and speak with a health professional to ensure their safety.
The TGA also said it did not regulate the advertising or provision of health services, with those matters falling under the remit of the Australian Health Practitioner Regulation Agency (AHPRA).
An AHPRA spokesperson said, in a statement, that legislation limited what the regulator could say about individual practitioners or matters, outside of information published on the register or in tribunal decisions.
Speaking generally, the regulator said disciplinary action had been taken against some practitioners, including cautions and conditions placed on their registration, over concerns about prescribing to vulnerable patients with disordered eating.
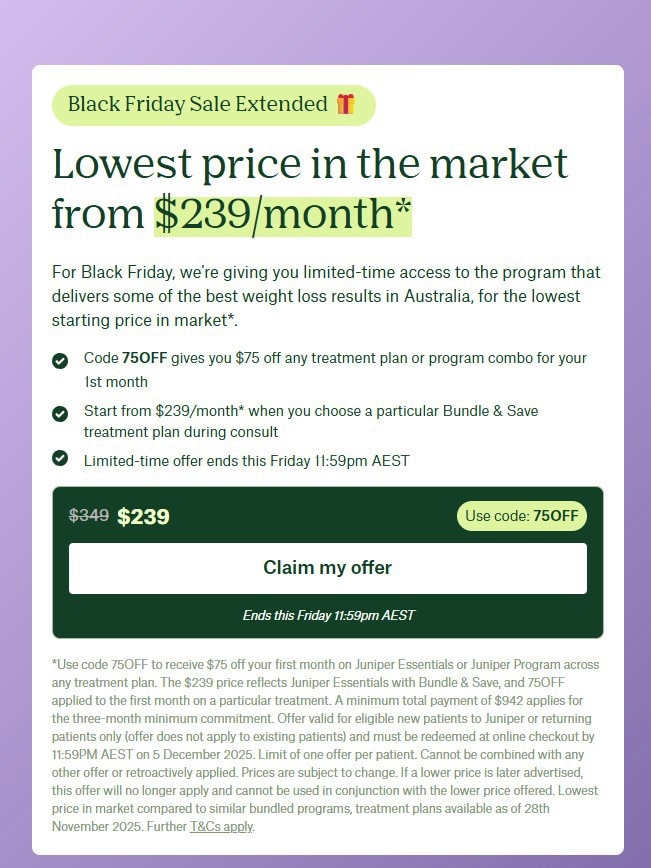
“We do not regulate companies or services, but our action against practitioners has also led telehealth providers to change their business practices.”
The TGA says the government could not comment on whether the companies were engaging in unlawful advertising of therapeutic goods. (ABC News: Shari Hams)
Comprehensive clinical safeguards
Dr Vickers said Juniper and Pilot had comprehensive clinical safeguards to ensure people with active or emerging eating disorders were not prescribed weight-management medications.
“Every patient is required to undertake a detailed medical questionnaire, followed by an in-depth phone or video consultation with an AHPRA-registered healthcare practitioner, who conducts a full history, risk assessment, and eligibility screen, including assessing BMI via verified video or photos, before any treatment is considered,” he said in a statement.
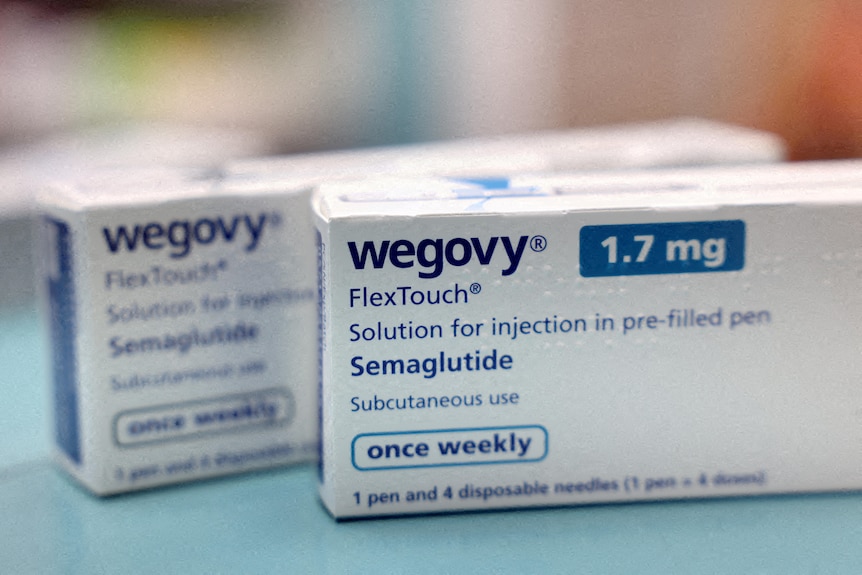
There are concerns people with restrictive eating disorders could access weight-loss treatments (Reuters: Hollie Adams)
“As part of our safeguard measures, we are also rolling out a live photo-verification system, further strengthening the clinical screening process. The system is scheduled to be fully implemented by the end of January 2026.”
Weight-loss drug manufacturers under fire for ‘unethical’ ads
He added that practitioners receive extra training to identify red flags, recognise early disordered eating, and manage weight stigma and body image concerns.
“Where concerns arise, medication is not prescribed, and patients are redirected to appropriate care and support,” the statement said.
“Ongoing monitoring of medical weight-loss patients, follow-up and access to multidisciplinary support form part of our broader care model to ensure that treatment remains clinically appropriate and safe.”
But eating disorder advocates said these measures were not enough and that in-person consultations should be mandated by law.
“What we are really concerned about is the ongoing medical monitoring of people who are taking these medications,” Ms Gooding said.
“What we really need is for our government to implement legislation for the safe prescribing of these medications.
“With these business models, follow-ups don’t always include the same doctor, and it certainly doesn’t include the same clinician. There’s a few things we are pushing for, that all appointments require a visual or face-to-face assessment.”