Economic UNDo, Affairs S. Revision of World Population Prospects. 2019.
Aktar W, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2009;2(1):1–12.
Hussain S, Siddique T, Saleem M, Arshad M, Khalid A. Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv Agron. 2009;102:159–200.
Soman C, Li D, Wander MM, Kent AD. Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil. 2017;413(1–2):145–59.
Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17(8):478–86.
Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60(4):579–98.
Balser TC, Firestone MK. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry. 2005;73(2):395–415.
Tian T, Reverdy A, She Q, Sun B, Chai Y. The role of rhizodeposits in shaping rhizomicrobiome. Environ Microbiol Rep. 2020;12(2):160–72.
Dennis PG, Miller AJ, Hirsch PR. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol. 2010;72(3):313–27.
Badri DV, Vivanco JM. Regulation and function of root exudates. Plant Cell Environ. 2009;32(6):666–81.
el Zahar Haichar F, Heulin T, Guyonnet JP, Achouak W. Stable isotope probing of carbon flow in the plant holobiont. Curr Opin Biotechnol. 2016;41:9–13.
Dakora FD, Phillips DA. Root exudates as mediators of mineral acquisition in low-nutrient environments. In: Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities. Springer; 2002. p. 201–13.
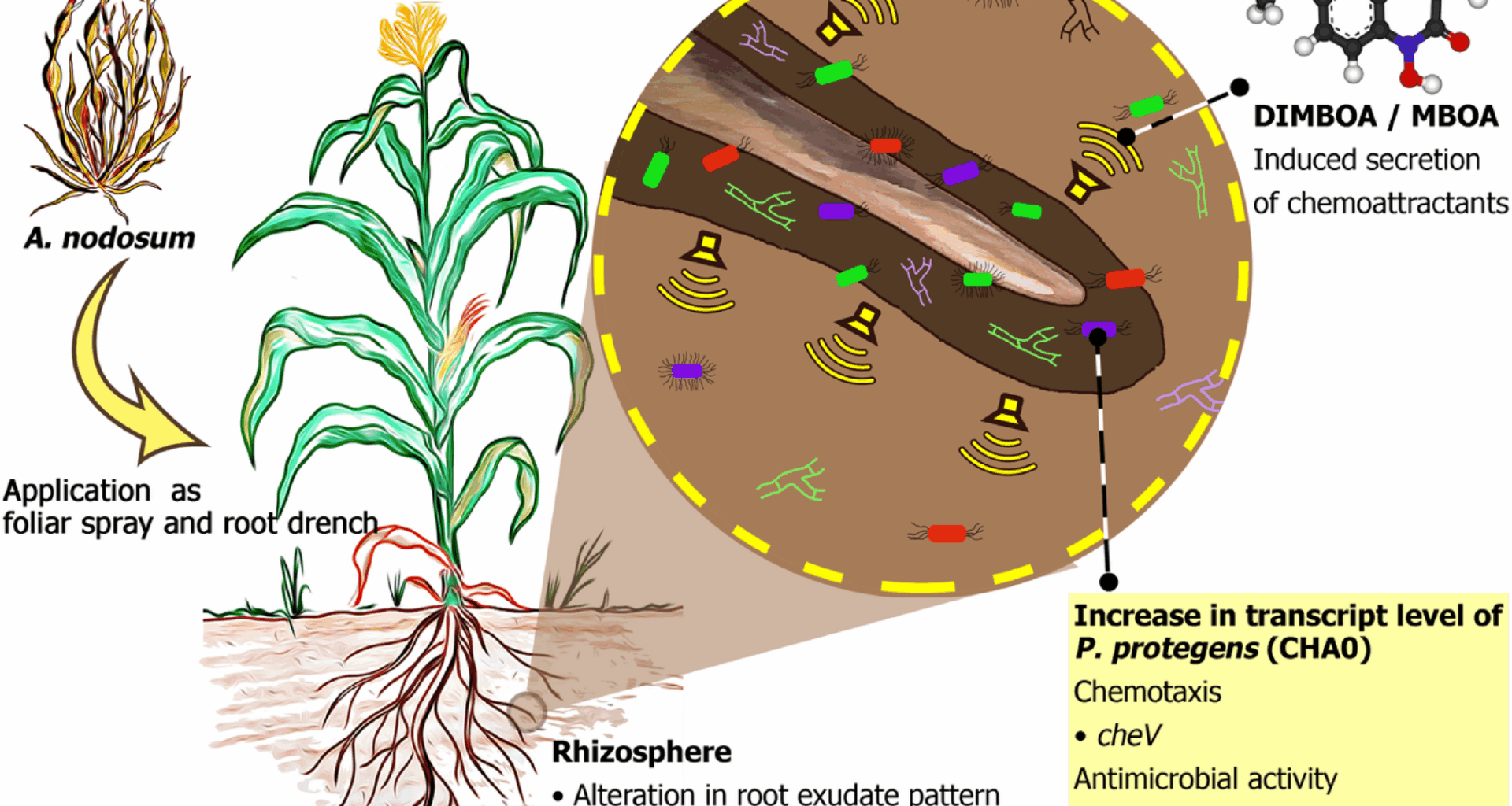
Neal AL, Ahmad S, Gordon-Weeks R, Ton J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS One. 2012;7(4):e35498.
Idris EE, Iglesias DJ, Talon M, Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Molecular Plant-Microbe Interactions®. 2007;20(6):619–26.
Persello-Cartieaux F, Nussaume L, Robaglia C. Tales from the underground: molecular plant–rhizobacteria interactions. Plant Cell Environ. 2003;26(2):189–99.
Islam S, Akanda AM, Prova A, Islam MT, Hossain MM. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 2016;6:1360.
Vinodkumar S, Nakkeeran S, Renukadevi P, Malathi V. Biocontrol potentials of antimicrobial peptide producing Bacillus species: multifaceted antagonists for the management of stem rot of carnation caused by Sclerotinia sclerotiorum. Front Microbiol. 2017;8: 446.
Vinodkumar S, Nakkeeran S, Renukadevi P, Mohankumar S. Diversity and antiviral potential of rhizospheric and endophytic Bacillus species and phyto-antiviral principles against tobacco streak virus in cotton. Agric Ecosyst Environ. 2018;267:42–51.
Tiwari S, Lata C, Chauhan PS, Nautiyal CS. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem. 2016;99:108–17.
García JE, Maroniche G, Creus C, Suárez-Rodríguez R, Ramirez-Trujillo JA, Groppa MD. In vitro PGPR properties and osmotic tolerance of different azospirillum native strains and their effects on growth of maize under drought stress. Microbiol Res. 2017;202:21–9.
Armada E, Azcón R, López-Castillo OM, Calvo-Polanco M, Ruiz-Lozano JM. Autochthonous arbuscular mycorrhizal fungi and Bacillus thuringiensis from a degraded mediterranean area can be used to improve physiological traits and performance of a plant of agronomic interest under drought conditions. Plant Physiol Biochem. 2015;90:64–74.
Ortiz N, Armada E, Duque E, Roldán A, Azcón R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. J Plant Physiol. 2015;174:87–96.
Cray JA, Connor MC, Stevenson A, Houghton JD, Rangel DE, Cooke LR, et al. Biocontrol agents promote growth of potato pathogens, depending on environmental conditions. Microb Biotechnol. 2016;9(3):330–54.
Simcock A. World Ocean Assessment. Cambridge University Press; 2017.
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, et al. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul. 2009;28(4):386–99.
Blunden G, Gordon SM. Betaines and their sulphonio analogues in marine algae. Progress Phycological Res. 1986;4:39–80.
Jayaraman J, Norrie J, Punja ZK. Commercial extract from the brown seaweed ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J Appl Phycol. 2011;23(3):353–61.
Bajpai S, Shukla PS, Asiedu S, Pruski K, Prithiviraj B. A biostimulant preparation of brown seaweed ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol J. 2019;35(5):406.
Cheng N, Peng Y, Kong Y, Li J, Sun C. Combined effects of biochar addition and nitrogen fertilizer reduction on the rhizosphere metabolomics of maize (Zea mays L.) seedlings. Plant Soil. 2018;433(1):19–35.
Pedersen HA, Heinrichson K, Fomsgaard IS. Alterations of the benzoxazinoid profiles of uninjured maize seedlings during freezing, storage, and lyophilization. J Agric Food Chem. 2017;65(20):4103–10.
de Bruijn WJ, Vincken J-P, Duran K, Gruppen H. Mass spectrometric characterization of benzoxazinoid glycosides from rhizopus-elicited wheat (Triticum aestivum) seedlings. J Agric Food Chem. 2016;64(32):6267–76.
Ling N, Raza W, Ma J, Huang Q, Shen Q. Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol. 2011;47(6):374–9.
Prithiviraj B, Kant P, Narayanan JM, Khan W, Hankins S, Neily W, et al. Bioactive compounds of ascophyllum nodosum and their use for alleviating salt-induced stress in plants. Google Patents; 2011.
Shukla PS, Borza T, Critchley AT, Hiltz D, Norrie J, Prithiviraj B. Ascophyllum nodosum extract mitigates salinity stress in Arabidopsis thaliana by modulating the expression of miRNA involved in stress tolerance and nutrient acquisition. PLoS One. 2018. https://doi.org/10.1371/journal.pone.0206221.
Rayirath P, Benkel B, Hodges DM, Allan-Wojtas P, MacKinnon S, Critchley AT, et al. Lipophilic components of the brown seaweed, ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta. 2009;230(1):135–47.
Santaniello A, Scartazza A, Gresta F, Loreti E, Biasone A, Di Tommaso D, et al. Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front Plant Sci. 2017;8:1362.
Łangowski Ł, Goñi O, Quille P, Stephenson P, Carmody N, Feeney E, et al. A plant biostimulant from the seaweed ascophyllum nodosum (Sealicit) reduces podshatter and yield loss in oilseed rape through modulation of IND expression. Sci Rep. 2019;9(1):1–11.
Jayaraj J, Wan A, Rahman M, Punja Z. Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot. 2008;27(10):1360–6.
Ali O, Ramsubhag A, Daniram Benn S Jr, Jayaraman J. Transcriptomic changes induced by applications of a commercial extract of Ascophyllum nodosum on tomato plants. Sci Rep. 2022;12(1):8042.
Villa e Vila V, Rezende R, Marques PAA, Wenneck GS, Nocchi RCF, Terassi DS, et al. Seaweed extract of ascophyllum nodosum applied in tomato crop as a biostimulant for improving growth, yield and soil fertility in subtropical condition. J Appl Phycol. 2023;35(5):2531–41.
Subramaniyan L, Veerasamy R, Prabhakaran J, Selvaraj A, Algarswamy S, Karuppasami KM, et al. Biostimulation effects of seaweed extract (Ascophyllum nodosum) on phytomorpho-physiological, yield, and quality traits of tomato (Solanum lycopersicum L). Horticulturae. 2023;9(3):348.
Zhang N, Wang D, Liu Y, Li S, Shen Q, Zhang R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil. 2014;374(1–2):689–700.
Rashid MH, Kornberg A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2000;97(9):4885–90.
Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–4.
Ziegler J, Schmidt S, Chutia R, Müller J, Böttcher C, Strehmel N, et al. Non-targeted profiling of semi-polar metabolites in Arabidopsis root exudates uncovers a role for coumarin secretion and lignification during the local response to phosphate limitation. J Exp Bot. 2016;67(5):1421–32.
Tan S, Yang C, Mei X, Shen S, Raza W, Shen Q, et al. The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl Soil Ecol. 2013;64:15–22.
Scher FM, Kloepper JW, Singleton CA. Chemotaxis of fluorescent Pseudomonas spp. to soybean seed exudates in vitro and in soil. Can J Microbiol. 1985;31(6):570–4.
Scher F, Kloepper J, Singleton C, Zaleska I, Laliberte M. Colonization of soybean roots by Pseudomonas and Serratia species: relationship to bacterial motility, chemotaxis, and generation time. Phytopathology. 1988;78(8):1055–9.
Chen Y, Cao S, Chai Y, Clardy J, Kolter R, Guo Jh, et al. A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol. 2012;85(3):418–30.
Kandasamy S, Khan W, Kulshreshtha G, Evans F, Critchley AT, Fitton J, et al. The fucose containing polymer (FCP) rich fraction of ascophyllum nodosum (L.) Le jol. Protects caenorhabditis elegans against Pseudomonas aeruginosa by triggering innate immune signaling pathways and suppression of pathogen virulence factors. Algae. 2015;30(2):147–61.
Sampedro I, Parales RE, Krell T, Hill JE. Pseudomonas chemotaxis. FEMS Microbiol Rev. 2014;39(1):17–46.
Ferrández A, Hawkins AC, Summerfield DT, Harwood CS. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J Bacteriol. 2002;184(16):4374–83.
Whitchurch CB, Leech AJ, Young MD, Kennedy D, Sargent JL, Bertrand JJ, et al. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol Microbiol. 2004;52(3):873–93.
Yao J, Allen C. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J Bacteriol. 2006;188(10):3697–708.
de Weert S, Vermeiren H, Mulders IH, Kuiper I, Hendrickx N, Bloemberg GV, et al. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant Microbe Interact. 2002;15(11):1173–80.
López-Farfán D, Reyes-Darias JA, Matilla-Vazquez MA, Krell T. Concentration dependent effect of plant root exudates on the chemosensory systems of Pseudomonas putida KT2440. Front Microbiol. 2019;10:78.
Yuan J, Wu Y, Zhao M, Wen T, Huang Q, Shen Q. Effect of phenolic acids from banana root exudates on root colonization and pathogen suppressive properties of Bacillus amyloliquefaciens NJN-6. Biol Control. 2018;125:131–7.
Garbeva P, Hordijk C, Gerards S, de Boer W. Volatile-mediated interactions between phylogenetically different soil bacteria. Front Microbiol. 2014;5:289.
Alam MZ, Braun G, Norrie J, Hodges DM. Effect of ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can J Plant Sci. 2013;93(1):23–36.
Alam MZ, Braun G, Norrie J, Mark Hodges D. Ascophyllum extract application can promote plant growth and root yield in Carrot associated with increased root-zone soil microbial activity. Can J Plant Sci. 2013;94(2):337–48.
Renaut S, Masse J, Norrie JP, Blal B, Hijri M. A commercial seaweed extract structured microbial communities associated with tomato and pepper roots and significantly increased crop yield. Microb Biotechnol. 2019;12(6):1346–58.
Kulshreshtha G, Borza T, Rathgeber B, Stratton GS, Thomas NA, Critchley A, et al. Red seaweeds sarcodiotheca gaudichaudii and chondrus crispus down regulate virulence factors of Salmonella enteritidis and induce immune responses in caenorhabditis elegans. Front Microbiol. 2016;7:421.
Kulshreshtha G, Rathgeber B, MacIsaac J, Boulianne M, Brigitte L, Stratton G, et al. Feed supplementation with red seaweeds, chondrus crispus and sarcodiotheca gaudichaudii, reduce Salmonella enteritidis in laying hens. Front Microbiol. 2017;8:567.
Liu J, Kandasamy S, Zhang J, Kirby CW, Karakach T, Hafting J, et al. Prebiotic effects of diet supplemented with the cultivated red seaweed Chondrus crispus or with fructo-oligo-saccharide on host immunity, colonic microbiota and gut microbial metabolites. BMC Complement Altern Med. 2015;15(1):279.
Visioli G, Sanangelantoni A, Vamerali T, Dal Cortivo C, Blandino M. 16S rDNA profiling to reveal the influence of seed-applied biostimulants on the rhizosphere of young maize plants. Molecules. 2018;23(6):1461.
Takahashi Y. Genus kitasatospora, taxonomic features and diversity of secondary metabolites. J Antibiot. 2017;70(5):506.
Mohapatra B, Sar P, Kazy SK, Maiti MK, Satyanarayana T. Taxonomy and physiology of Pseudoxanthomonas arseniciresistens sp. nov., an arsenate and nitrate-reducing novel gammaproteobacterium from arsenic contaminated groundwater, India. PLoS One. 2018;13(3):e0193718.
Rangjaroen C, Sungthong R, Rerkasem B, Teaumroong N, Noisangiam R, Lumyong S. Untapped endophytic colonization and plant growth-promoting potential of the genus Novosphingobium to optimize rice cultivation. Microbes Environ. 2017;32(1):84–7.
Niemeyer HM. Hydroxamic acids derived from 2-hydroxy-2 H-1, 4-benzoxazin-3 (4 H)-one: key defense chemicals of cereals. J Agric Food Chem. 2009;57(5):1677–96.
Kudjordjie EN, Sapkota R, Steffensen SK, Fomsgaard IS, Nicolaisen M. Maize synthesized benzoxazinoids affect the host associated microbiome. Microbiome. 2019;7(1):59.
Oliveros-Bastidas A, Molinillo JM, Macias FA, Chinchilla N. Absorption and elimination of the allelochemical MBOA by weeds during seedling growth. Agronomy. 2021;11(3): 471.
Yang M, Zhang YU, Qi L, Mei X, Liao J, Ding X, Deng W, Fan L, He X, Vivanco JM, Li C. Plant-plant-microbe mechanisms involved in soil-borne disease suppression on a maize and pepper intercropping system. PLoS One. 2014;9(12): e115052.
Sicker D, Schulz M. Benzoxazinones in plants: occurrence, synthetic access, and biological activity. Studies in natural products chemistry, vol. 27. Elsevier; 2002. p. 185–232.
Fomsgaard IS, Mortensen AG, Carlsen SC. Microbial transformation products of benzoxazolinone and benzoxazinone allelochemicals––a review. Chemosphere. 2004;54(8):1025–38.