Reagents and equipment
Beef extract and bacteriological peptone purchased from Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., dextrose purchased from Xilong Science Co., Ltd., and agar powder purchased from Beijing Solarbio Science & Technology Co., Ltd., were used for the preparation of culture media. A copper hydroxide water particle dispersant (Kocide 3000, 46% metallic copper) was purchased from Corteva Agriscience (Shanghai, China), and used as the positive control. Chromatographic reagents petroleum ether (PE), ethyl acetate (EtOAc), n-butanol, acetone, dichloromethane, and methanol were of analytical grade, obtained from Xilong Science Co., Ltd, Sichuan, China. Silica gel (100 − 300 mesh, Qingdao Marine Chemical Inc., Qingdao, China) and Sephadex LH-20 (Amersham Biosciences Inc., Shanghai, China) were used for column chromatography. Analytical thin-layer chromatography (TLC) was performed on silica gel GF254 plates (Qingdao Marine Chemical Inc., Qingdao, China). The other chemicals, absolute alcohol, NaCl, KCl, Na2HPO4, and KH2PO4 obtained from Xilong Science Co., Ltd., and 2.5% glutaraldehyde fixing solution obtained from Shanghai Macklin Biochemical Technology Co., Ltd, were all of analytical grade, used for the preparation of scanning electron microscope samples. Optical rotations were measured at the sodium D line (589 nm) on a WXG-4 disc polarimeter [INESA (Group) Co., Ltd., Shanghai, China]. HRESI-MS/MS spectrum was measured using an Esquire HCT ion trap mass spectrometer (Bruker Daltonics Inc. Billerica, USA) using negative ion mode. NMR spectra were recorded on a Bruker ADVANCE III 500 spectrometer (Bruker, Karlsruhe, Germany). High-performance liquid chromatography (HPLC) analysis was conducted using a Waters e2695-ELS detector (Waters Corporation, Massachusett, USA) with a 4.6 mm × 250 mm i.d. and a 5 μm Agilent Zorbax SB-C18 column (Agilent Technologies Inc., California, USA). A Buchi MPLC system (C-615) (BUCHI, Switzerland) with a 250 mm × 10 mm i.d. and a 10 μm COSMOSIL C18 column (Nacalai Tesque Inc., Kyoto, Japan) was used for MPLC. Fluorescence microplate read (SpectraMax i3x Platform, Molecular Devices) was used for the determination of the OD value of bacterial suspension. A scanning electron microscope (SEM) (CLARA, Navi, TESCAN) was used for observation of the cell morphology.
Microbial materials
The pathogen Xanthomonas citri subsp. citri (Xcc) of citrus canker disease used in this study was provided by the Chemical Ecology Laboratory of the Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection, Guangxi Normal University. The EF Nemania sp. LJZ-Y-11 (GenBank accession number: MK351455) was previously isolated from healthy leaves of Citrus reticulata Blanco cv. Shatangju [19].
Citrus plants
Citrus reticulata ‘Orah orange’ provided by Guangxi Institute of Specialty Crops, was used for determining the effects of the active compound isolated from Nemania sp. LJZ-Y-11 on the Xcc in vivo. The new leaves nearly mature were chosen for the inoculation.
Culture media
Nutrient agar culture medium (NA) containing 3 g/L of beef extract, 5 g/L of bacteriological peptone, 2.5 g/L of dextrose, and 18 g/L of agar powder, with pH 7.0, was used for the activation culture of Xcc from low-temperature storage tube and the spread plate for the validation of the minimum bactericidal concentration (MBC).
Nutrient in both medium (NB) containing 3 g/L of beef extract, 5 g/L of bacteriological peptone, and 2.5 g/L of dextrose, with pH 7.0, was used for Xcc culture and the preparation of bacterial suspension.
Potato dextrose agar medium (PDA) containing 200 g/L of potato (peeled), 20 g/L of dextrose, 15–20 g/L of agar powder, with pH 7.0, was used for the activation culture of EF Nemania sp. LZJ-Y-11.
Potato dextrose broth medium (PDB) containing 200 g/L of potato (peeled), and 20 g/L of dextrose, with pH 7.0, was used for the culture of EF Nemania sp. LZJ-Y-11 to prepare the seed liquid.
Rice solid culture medium was prepared as follows: rice and water were 1/1 (m/v), with 100 g of rice per 1000 mL flask. The medium was used for the fermentation culture of EF Nemania sp. LZJ-Y-11.
Fermentation culture of the EF Nemania sp. LZJ-Y-11 and the preparation of extracts
According to a previous method with some minor modifications, the EF Nemania sp. LZJ-Y-11 was fermented to prepare extracts [19]. The EF Nemania sp. LZJ-Y-11 was firstly cultured on the PDA plate at 27 ± 1 °C for 5 days. Then three plugs from the edge of the actively growing colony were inoculated into a 250-mL Erlenmeyer flask containing 100 mL of PDB; the inoculated medium was then incubated on a rotary shaker at 150 rpm and 27 ± 1 °C for 3–5 days. Next, 5 mL of the mycelial suspension was inoculated into a 1000-mL Erlenmeyer flask containing 100 g of rice grain (autoclaved). All flasks were incubated under static conditions, in the dark, at 27 ± 1 °C for 30–45 days. After fermentation, the rice grains, which were covered with profuse fungal growth, were dried and powdered and then were used to prepare an extract using ultrasonication with EtOAc three times. The three filtrates were merged and evaporated to dryness and the crude extract (CE) was obtained. For the preliminary separation, the CE was dissolved in water, and then extracted three times with an equal volume of PE, EtOAc, and n-butanol, respectively. The three times filtrates were also merged for each solvent. The remaining water and the merged filtrates were evaporated to dryness to obtain the extracts of water residue, PE, EtOAc, and n-butanol, respectively.
Isolation and identification of compounds from EF Nemania sp. LZJ-Y-11
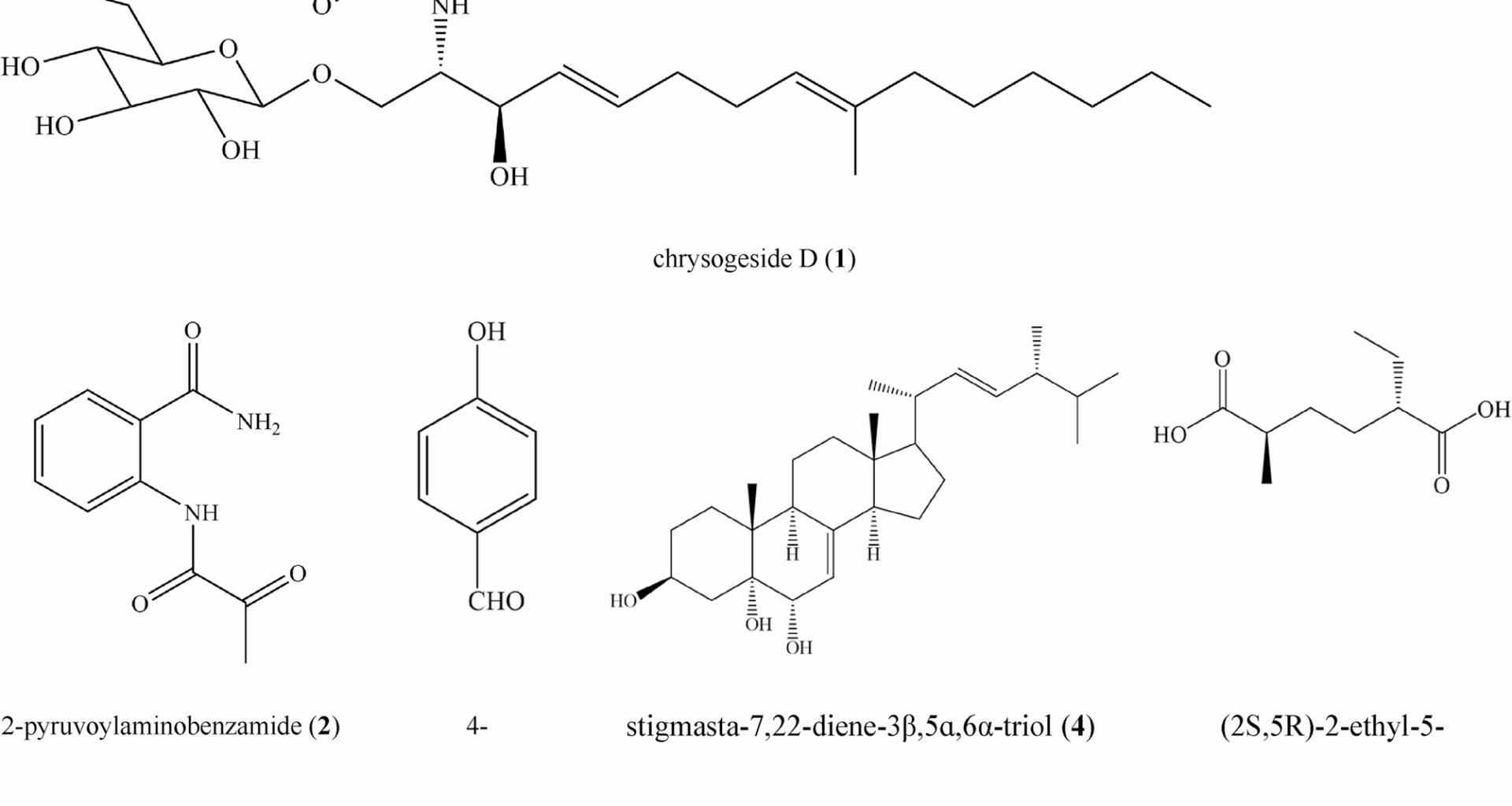
By a bioassay guiding, the EtOAc extract of Nemania sp. LZJ-Y-11 was fractionated using silica gel column chromatography with a dichloromethane/methanol mixture (20/1, v/v); we obtained five fractions (Fr. I-V), which were monitored and pooled using TLC analysis. Fr. III was subjected to silica gel chromatography using mixtures of petroleum ether/acetone with increasing polarity (15/1, 10/1, 5/1, 1/1, 0/1, v/v) as eluents to yield 12 subfractions (Frs. 1 to 12). Next, Frs. nine (5 g) was subjected to MPLC (MeOH/H2O, 1/5→1/1, v/v) to yield seven subfractions (S1 to S7). S1 (236.0 mg) was purified over a Sephadex LH-20 column (methanol) and chromatographed over silica gel (dichloromethane/methanol, 30/1, v/v) to obtain compound 1 (29 mg). S4 (263.0 mg) was purified over a Sephadex LH-20 column (methanol) and chromatographed over silica gel (dichloromethane/methanol, 45/1, v/v) to obtain compound 2 (20 mg), and S4 (192.0 mg) was purified using HPLC (C18, methanol/water, 7/20–4/5, v/v) to obtain compound 3 (18 mg). S5 (142.0 mg) was purified over a Sephadex LH-20 column (methanol) and chromatographed over silica gel (dichloromethane/methanol, 45/1, v/v) to obtain compound 4 (7 mg). S7 (2519.1 mg) was purified over a Sephadex LH-20 column (dichloromethane/methanol, 1/1, v/v) and chromatographed over silica gel (petroleum ether/acetone + 0.1% acid, 8/1→5/1→3/1, v/v) to obtain compound 5 (937.5 mg).
The culture of Xanthomonas citri subsp. citri and the preparation of suspension
Xcc preserved at low temperature was firstly inoculated onto an NA plate, and incubated at 27 ± 1℃ for 3 days, repeated transfer once. Then the bacteria were inoculated into a 250-mL Erlenmeyer flask containing 100 mL of NB medium, cultured at 27 ± 1 ℃, 150 rpm for 24 h, to obtain the Xcc suspension liquid (OD value = 0.19, plate count of 109 CFU/mL). The suspension was diluted 100 times to obtain a bacterial suspension with a concentration of 107 CFU/mL and used for the determination of antibacterial activity and the growth curve assay.
Determination of the antibacterial activity
The antibacterial activity of secondary metabolites from EF Nemania sp. LZJ-Y-11 against Xcc was determined as follows. The required concentration of extracts or compounds was dissolved in acetone/water (1/1, v/v) to prepare sample solutions. Then 0.2 mL of the sample solution was evenly mixed with 1.6 mL of NB medium and 0.2 mL of bacterial suspension (prepared as the above method 1.7) in a tube, and the acetone/water (1/1, v/v) was used in the same way to prepare a control. Three replicates were set for each treatment and incubated at 27 ± 1℃ in a constant temperature shaking incubator at 150 rpm for 24, 48, and 72 h to observe bacterial growth. The MIC was the minimum concentration at which there was no bacterial growth during 72 h. For the MBC, 50 µL of solutions of the MIC and the higher concentrations were spread on NA plates (in the Petri dish with a diameter of 6 cm), and then incubated at 27 ± 1℃. The growth of Xcc was observed at 24, 48, and 72 h, and the lowest concentration at which there was no growth of bacteria on the NA medium for 72 h was recorded as the MBC.
The growth curve assay
The effect of the active compound on Xcc growth in vitro was evaluated by the growth curve assay [30]. The culture of Xcc and the seed suspension were prepared as described in the above method 1.7, and the seed suspension (OD600 = 1.9, ≈109 CFU/mL) was diluted 100 times to obtain a bacterial suspension with a concentration of 107 CFU/mL and used for the growth curve assay. This bacterial suspension (2 mL) and NB (16 mL) were added into a 50 mL conical flask, followed by the addition of 2 mL of compound solution (acetone/water, 1/1, v/v, as the solvent, prepared as described in the method 1.8) at series concentrations to achieve a final concentration of 0.03125, 0.06125, 0.125, 0.25, and 0.5 mg.mL−1, respectively. The conical flasks were then incubated at 150 rpm and 27 ± 1 °C. Two hundred microliters of incubation solution in the conical flasks were taken out and put into a 96-well plate at the incubation times of 0 h, 8 h, 24 h, 32 h, 48 h, 56 h, and 72 h, respectively. The absorbance of the incubation solution was measured at OD600 nm. The mixture of acetone/water (1/1, v/v) prepared in the same way was used as a control. All measurements were performed in triplicate and each experiment contained three replications. The Xcc growth curve was plotted by incubation time versus the OD600 values of the incubation solution.
Scanning electron microscopy (SEM) studies
The effects of the active compound on the Xcc cell morphology were observed by using the SEM [30, 31]. Referring to the above method in 1.7, Xcc was incubated for 24 h (OD600 = 0.19, ≈109 CFU/mL), then 1 mL of the bacterial solution was taken into a 2 mL centrifuge tube and centrifuged at 5000 rpm for 5 min. The supernatant was removed to obtain the bacterial cells, and 0.9 mL of NB medium was added and mixed evenly. The mixture was transferred to a glass tube (1.5 × 10 cm in diameter and length), and 0.1 mL of the sample solution was added to obtain the mixture containing the sample for the final concentrations of 0 (control), 0.03125, 0.0625, 0.125, 0.25, and 0.5 mg.mL−1, with 3 replicates for each treatment. After being incubated in a shaking incubator at 27 ± 1℃ and 150 rpm for 10 h, the mixture was centrifuged (5000 rpm, 10 min) to collect the bacterial cells. The bacterial cells were washed twice with PBS solution (pH = 7.0). The bacterial cells were collected by centrifugation, and then, 500 µL glutaraldehyde with a concentration of 2.5% was added and mixed well, fixed at low temperature in the dark at 4℃ for 4 h. Next, the bacterial cells were collected by centrifuging at 5000 rpm for 10 min and washed twice with PBS solution with a pH of 7.0. The bacterial cells were collected by centrifugation. Then dehydrated with 30%, 50%, 70%, 80%, 90%, and anhydrous ethanol in sequence, by standing for 10 min respectively, and the supernatant was removed. An appropriate amount of anhydrous ethanol was added to the bacterial cells and mixed evenly to prepare a bacterial suspension. 1 − 2 µL of the bacterial suspension was dropped onto the mirror surface of the silicon wafer and gold-plated three times, with a duration of 1 min for each time. After the gold-plating was completed, SEM observation was performed.
Attached leaf assay
The effect of the active compound on Xcc in vivo was determined by using an attached leaf assay, referring to Llorens et al. [8], with some modifications, i.e., the Xcc suspension was inoculated into the attached leaves of the citrus plant. The bacterial suspension (0.2 mL, 107 CFU/mL, prepared as the above method 1.7) and NB (1.6 mL) were added into a glass tube, followed by the addition of 0.2 mL of compound solution (prepared as described in the method 1.8) to achieve a final concentration of 0.03125, 0.06125, 0.125, 0.25, and 0.5 mg.mL−1, respectively. An equal volume of acetone/water (1/1, v/v) was prepared as the control group. Three replicates were set for each group, and all measurements were incubated at 27 ± 1℃ and 150 rpm for 72 h. The culture solution was inoculated into citrus leaves using a previous method with slight modifications [8, 32]. The selected leaves that were newly grown, had reached their maximum leaf area, had just turned green, had grown well, and had not suffered any damage from inoculation. The bacterial solution was injected with a 1-mL sterile needleless syringe on the abaxial leaf blade with approximately 2 µL of bacterial suspension at each injection, and 3 injections were performed on each side of the leaf mid-vein, i.e., a total of 6 injections per leaf for each treatment. Three leaves were inoculated for each sample. Lesion expansion and symptom development were recorded periodically after inoculation, and the photos were taken on the 25th day post-inoculation.
Statistical analysis
All experiments were performed in triplicate. Statistical analyses, including graphical representations, were performed using Microsoft Excel 2016 (Microsoft Corporation).