Bangladesh
According to the Institute of Epidemiology Disease Control and Research (IEDCR) in Dhaka, four confirmed Nipah virus cases were reported in Bangladesh in 2025, accounting for 4 fatalities.
The four confirmed cases were reported from four different districts across three separated geographical divisions (Barisal, Dhaka, and Rajshahi) in Bangladesh.
According to the WHO, all cases were confirmed through Reverse Transcription Polymerase Chain Reaction (PCR) and Enzyme-Linked Immunosorbent Assay (ELISA) testing, and no epidemiological links were reported to have been identified between the cases.
The first case was a young adult woman from Pabna district, Rajshahi division, with symptom onset on 25 January. She was admitted to a community hospital on 26 January and referred to another hospital the next day. She died on 28 January, and laboratory confirmation of NiV was received on 29 January. A total of 96 contacts were reported to be identified, and all tested negative for NiV.
The second case was an adult man from Bhola district, Barisal division, who developed symptoms on 13 February and was admitted to hospital on 19 February. He was transferred to another hospital the next day and died on 22 February. NiV infection was confirmed on 21 February. A total of 71 contacts were reportedly identified, and all tested negative for NiV.
The third case was an adult man from Faridpur district, Dhaka division, with symptom onset on 17 February. He was admitted to hospital on 25 February and died the same day. NiV infection was confirmed on 26 February. A total of 66 contacts were identified, and all tested negative for NiV.
The fourth case was a male child from Naogaon district, Rajshahi division, with symptom onset on 3 August. He was admitted to a hospital on 8 August and moved to the intensive care unit the following day. He died on 14 August. Samples collected on 10 August tested positive for NiV on 22 August. An outbreak investigation team was deployed the same day. A total of 72 contacts were identified, and samples from 11 symptomatic contacts were collected. Six tested negative, while the results for the remaining are awaited. This case was reported outside the typical season (December to April).
The first three cases had a history of consuming raw palm sap. However, the fourth case had no history of consuming raw palm sap, and the likely source/s of infection remain under investigation.
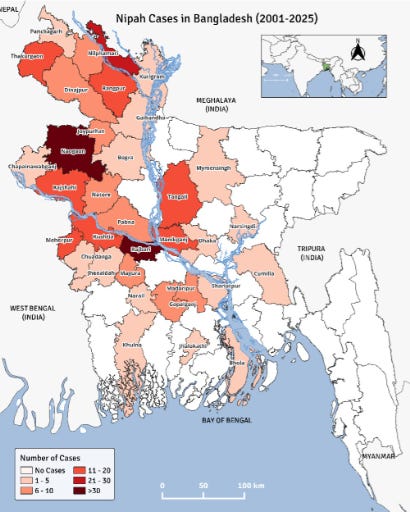
Since 2001, IEDCR has reported 347 Nipah virus infections, including 249 deaths, for a case fatality of 71.8 percent.
The worst year was 2004 when 67 cases and 50 deaths (75% CFR). Three years saw no cases-2002, 2006 and 2016.
Nipah virus (NiV) is an emerging and serious viral zoonotic disease that carries with it a high case fatality rate (in the ballpark of 70% or more).
NiV is an enveloped RNA virus and along with Hendra virus make up the Henipahviruses.
The natural host of the virus are fruit bats of the Pteropodidae family, Pteropus genus.
It was first recognized in a large outbreak in Kampung Sungai Nipah, Malaysia and Singapore from Sep 1998 – May 1999.
276 cases were reported, the vast majority (93%) being pig farmers or people who had contact with pigs. The disease presented as encephalitis and four out 10 people died.
It is theorized that the pigs got infected consuming partially bat-eaten fruit that ended up in a pigsty. Pigs were the intermediate host in this case; however, subsequent outbreaks had no intermediate hosts.
No new outbreaks have been reported in Malaysia since 1999.
The outbreaks since the initial one in Malaysia (Bangladesh and India) have been linked to two possible routes of transmission—consumption of raw date palm sap that had been contaminated with urine or saliva from infected fruit bats and strong evidence points to human-to-human transmission (close physical contact, especially contact with body fluids).
Nipah vaccine
The University of Oxford has launched the world’s first Phase II clinical trial of a Nipah virus vaccine candidate.
The trial, conducted in Bangladesh in partnership with the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), and funded by the Coalition for Epidemic Preparedness Innovations (CEPI), will assess the safety and immune response of the ChAdOx1 NipahB vaccine in a region where the virus causes recurrent outbreaks.
The trial started earlier this month, and will enrol 306 healthy participants aged 18 to 55.
Developed by scientists at the University of Oxford’s Pandemic Sciences Institute (PSI), the first-in-human trials of the ChAdOx1 NipahB vaccine started in January 2024 in Oxford, led by the Oxford Vaccine Group. Fifty-one people aged 18 to 55 have safely completed one year of follow-up in the Oxford trial with results expected in the coming months.
The vaccine is made using the same viral vector platform as the Oxford/AstraZeneca COVID-19 vaccine, which is estimated to have saved six million lives in its first year alone.
Dr Kent Kester, CEPI’s Executive Director of Vaccine Research and Development, said: ‘Oxford’s Nipah virus vaccine candidate is the most advanced vaccine against this highly lethal virus. The start of this phase II trial is a first of its kind and represents the culmination of years of cutting-edge research and global scientific collaboration.
‘The results from this study will hopefully bring us a step closer towards protecting vulnerable populations against future deadly Nipah outbreaks and will help inform the development of other Paramyxovirus countermeasures.’