Solid Phase Carrier Resin for Peptide Drug Synthesis Market Forecast and Outlook (2025-2035)
The solid phase carrier resin for peptide drug synthesis market, expected to grow from USD 145.3 million in 2025 to USD 397.8 million by 2035 at a CAGR of 10.5%, is undergoing innovation cycles that directly influence efficiency, scalability, and therapeutic outcomes. A primary area of innovation is in resin design, where manufacturers are focusing on controlled pore size, optimized functional group density, and chemically stable backbones to enable longer peptide chains with reduced aggregation. Advances in linker chemistry are also enhancing the release efficiency of peptides from resins, minimizing by-products and improving overall yield in synthesis operations. These developments are closely tied to the increasing demand for high-purity peptides in both drug discovery and large-scale commercial production.
Automation-driven peptide synthesizers are further shaping innovation, as carrier resins are being engineered for compatibility with high-throughput systems. This shift is enabling contract manufacturing organizations and pharmaceutical firms to scale peptide production with minimal variability, aligning with stricter regulatory requirements for complex biologics. Another emerging trend lies in the creation of hybrid resins that combine organic and inorganic frameworks, delivering enhanced swelling properties and solvent compatibility. Such improvements are particularly significant for complex or highly hydrophobic peptides, where traditional resins often limit synthesis success.
Environmental and cost-efficiency concerns are also prompting the development of recyclable and reusable resins, aimed at reducing synthesis waste and operational expenditure. Academic research institutions are playing a vital role in piloting these innovations, while biotechnology firms are translating them into commercial-grade solutions. The growth trajectory suggests that peptide drug developers will increasingly rely on differentiated resin technologies not only for drug candidates targeting oncology, metabolic disorders, and infectious diseases but also for advanced therapeutic modalities like peptide–drug conjugates. The next wave of innovation is expected to emphasize integration with digital process control systems, allowing real-time monitoring of resin performance during synthesis.
Quick Stats for Solid Phase Carrier Resin for Peptide Drug Synthesis Market
Solid Phase Carrier Resin Market Value (2025): USD 145.3 million
Solid Phase Carrier Resin Market Forecast Value (2035): USD 397.8 million
Solid Phase Carrier Resin Market Forecast CAGR: 10.6%
Leading Resin Type in Market: Hydroxyl Resins
Key Growth Regions: East Asia, North America, and Western Europe
Key Players: Sunresin, Shandong Lukang, Tianjin Nankai Hecheng Science & Technology, Zhejiang Zhengguang
Where revenue comes from — now vs next (industry-level view)
Period
Primary Revenue Buckets
Share
Notes
Today
Hydroxyl resins (orthopedic applications)
42%
Traditional chemistry, established pharmaceutical applications
Chloromethyl resins (cardiovascular)
31%
Versatile linker chemistry, broad therapeutic use
Amino resins (metabolic diseases)
19%
Specialized applications, diabetes and obesity treatments
Others (tumor applications)
8%
Emerging oncology peptides, research applications
Future (3-5 yrs)
Advanced hydroxyl systems
38-41%
Enhanced loading capacity, improved purity
High-performance chloromethyl
26-29%
Optimized synthesis efficiency, automated compatibility
Specialized amino resins
16-19%
Novel metabolic targets, precision medicine
Oncology-focused resins
12-15%
Cancer immunotherapy, targeted drug delivery
Custom therapeutic resins
6-9%
Rare diseases, personalized medicine applications
Research & development
4-7%
Academic institutions, early-stage drug discovery
Solid Phase Carrier Resin for Peptide Drug Synthesis Market Key Takeaways
Metric
Value
Market Value (2025)
USD 145.3 million
Market Forecast (2035)
USD 397.8 million
Growth Rate
10.6% CAGR
Leading Resin Type
Hydroxyl Resins
Primary Application
Orthopedics Segment
The solid phase carrier resin for peptide drug synthesis market demonstrates strong fundamentals, with hydroxyl resin systems capturing a dominant share through advanced linker chemistry and pharmaceutical application optimization. Orthopedic applications drive primary demand, supported by the increasing development of therapeutic peptides and modernization initiatives within the pharmaceutical industry. Geographic expansion remains concentrated in developed markets with established pharmaceutical infrastructure, while emerging economies show accelerating adoption rates driven by biotechnology sector growth and rising research investments.
Imperatives for Stakeholders in Solid Phase Carrier Resin for Peptide Drug Synthesis Market Design for synthesis efficiency, not just capacity
Offer complete resin packages: carrier resins + linker systems + cleavage reagents + synthesis protocols + analytical guides + purification instructions.
Preconfigured synthesis workflows: automated protocols, yield optimization, purity monitoring, and digital synthesis management for pharmaceutical operations.
Pharmaceutical integration readiness
Real-time synthesis monitoring, resin performance tracking, and smart pharmaceutical integration (LIMS connectivity, batch management systems).
Quality-by-design approach
Advanced resin manufacturing systems, real-time quality monitoring, synthesis performance integration, and comprehensive peptide documentation.
Value-based pricing models
Clear base resin price + transparent service tiers (synthesis consulting, method development, technical support); subscriptions for resin supply management services.
Segmental Analysis
Primary Classification: The solid phase carrier resin for peptide drug synthesis market segments by resin type into hydroxyl, chloromethyl, amino, and others, representing the evolution from traditional solid-phase synthesis chemistry to sophisticated linker technologies for comprehensive peptide synthesis optimization.
Secondary Classification: Application segmentation divides the solid phase carrier resin for peptide drug synthesis market into orthopedics, cardiovascular, metabolic diseases, tumors, and others, reflecting distinct requirements for therapeutic peptides, synthesis efficiency, and pharmaceutical development specifications.
Tertiary Classification: End-use segmentation covers pharmaceutical companies, biotechnology firms, contract research organizations, academic institutions, and specialty chemical manufacturers, while distribution channels span direct sales, chemical distributors, and specialized pharmaceutical suppliers.
Regional Classification: Geographic distribution covers North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, and Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by pharmaceutical industry modernization programs.
The segmentation structure reveals resin progression from traditional hydroxyl chemistry toward sophisticated amino and specialty resins with enhanced synthesis capabilities, while application diversity spans from established orthopedic peptides to emerging oncology therapeutics requiring precision synthesis solutions.
By Resin Type, the Hydroxyl Segment Accounts for Dominant Market Share
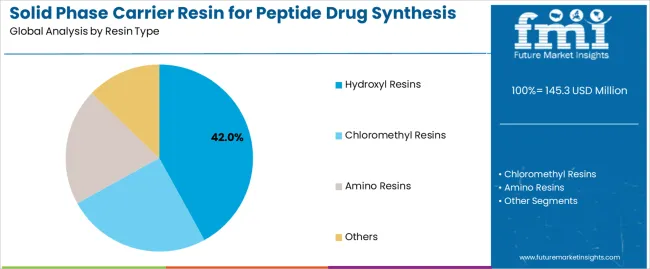
Market Position: Hydroxyl resin systems command the leading position in the solid phase carrier resin market with 42% market share through proven linker technologies, including efficient coupling chemistry, high loading capacity, and synthesis reliability that enable pharmaceutical manufacturers to achieve optimal peptide production across diverse orthopedic and therapeutic applications.
Value Drivers: The segment benefits from pharmaceutical industry preference for established resin systems that provide reliable synthesis performance, consistent peptide quality, and operational compatibility without requiring specialized synthesis infrastructure. Advanced hydroxyl chemistry features enable enhanced coupling efficiency, improved peptide purity, and integration with existing synthesis platforms, where performance reliability and therapeutic effectiveness represent critical manufacturing requirements.
Competitive Advantages: Hydroxyl resin systems differentiate through proven synthesis reliability, versatile linker compatibility, and integration with established peptide manufacturing systems that enhance facility effectiveness while maintaining optimal pharmaceutical standards suitable for diverse therapeutic applications.
Key market characteristics:
Advanced resin designs with optimized loading capacity and synthesis reliability capabilities
Enhanced coupling effectiveness, enabling 94-97% synthesis efficiency with reliable peptide production
Pharmaceutical compatibility, including automated synthesis systems, purity optimization, and quality control for therapeutic applications
Chloromethyl Shows Versatility Market Position
Chloromethyl resin systems maintain a 31% market position in the solid phase carrier resin market due to their versatility advantages and broad therapeutic application benefits. These materials appeal to facilities requiring flexible synthesis solutions with enhanced coupling profiles for diverse peptide sequences. Market growth is driven by cardiovascular peptide expansion, emphasizing versatile linker solutions and synthetic efficiency through optimized coupling designs.
By Application, the Orthopedics Segment Shows Strong Growth
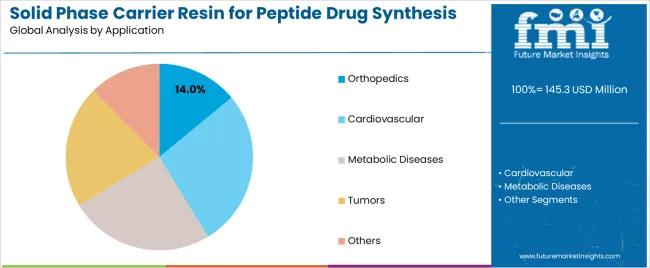
Market Context: Orthopedic applications demonstrate strong growth in the solid phase carrier resin market with 14% market share due to widespread adoption of therapeutic peptide programs and increasing focus on bone and joint treatments, operational synthesis efficiency, and peptide therapy applications that maximize therapeutic effectiveness while maintaining pharmaceutical standards.
Appeal Factors: Pharmaceutical operators prioritize synthesis reliability, therapeutic consistency, and integration with existing drug development infrastructure that enables coordinated peptide operations across multiple therapeutic programs. The segment benefits from substantial pharmaceutical industry investment and orthopedic research programs that emphasize the acquisition of premium resins for peptide differentiation and therapeutic outcome applications.
Growth Drivers: Orthopedic peptide development programs incorporate carrier resins as essential components for therapeutic synthesis, while specialized pharmaceutical growth increases demand for synthesis capabilities that comply with regulatory standards and minimize development complexity.
Market Challenges: Varying therapeutic requirements and regulatory complexity may limit resin standardization across different peptides or synthesis scenarios.
Application dynamics include:
Strong growth in bone regeneration and joint therapy peptides requiring premium synthesis capabilities
Increasing adoption in orthopedic biotechnology applications for pharmaceutical developers
Rising integration with synthesis automation systems for yield optimization and quality assurance
Cardiovascular Applications Maintain Therapeutic Demand
Cardiovascular applications capture 31% market share through specialized therapeutic requirements in cardiac peptides, vascular treatments, and pharmaceutical applications. These facilities demand premium resins capable of supporting regulatory requirements while providing therapeutic synthesis access and pharmaceutical development capabilities.
Metabolic Diseases Applications Show Specialized Growth
Metabolic diseases applications account for 19% market share, including diabetes treatments, obesity therapeutics, and endocrine applications requiring performance resin capabilities for therapeutic optimization and pharmaceutical effectiveness.
By End-Use, Pharmaceutical Companies Drive Market Leadership
Market Context: Pharmaceutical Companies dominate the solid phase carrier resin for peptide drug synthesis market with an 8.2% CAGR, reflecting the primary demand source for carrier resin technology in therapeutic peptide applications and drug development standardization.
Business Model Advantages: Pharmaceutical Companies provide direct market demand for standardized resin systems, driving volume production and cost optimization while maintaining quality control and regulatory compliance requirements.
Operational Benefits: Pharmaceutical Company applications include therapeutic standardization, synthesis efficiency, and quality assurance that drive consistent demand for resin systems while providing access to the latest synthesis technologies.
What are the Drivers, Restraints, and Key Trends of the Solid Phase Carrier Resin for Peptide Drug Synthesis Market?
Category
Factor
Impact
Why It Matters
Driver
Pharmaceutical industry growth & peptide drug development (therapeutic peptides, biologics expansion)
★★★★★
Growing pharmaceutical market requires specialized resins with enhanced synthesis capabilities and purity properties proven effective across therapeutic applications.
Driver
Therapeutic standards advancement & regulatory requirements (FDA approval, GMP compliance)
★★★★★
Transforms resin requirements from “basic chemistry” to “pharmaceutical grade synthesis”; manufacturers that offer quality resins and compliance features gain competitive advantage.
Driver
Biotechnology market growth & precision medicine (personalized therapy, targeted treatments)
★★★★☆
Biotechnology companies need sophisticated, high-performance resins; demand for specialized and superior synthesis solutions expanding addressable market.
Restraint
Cost pressures & development constraints (especially for small biotech companies)
★★★★☆
Smaller pharmaceutical developers defer resin upgrades; increases price sensitivity and slows premium resin adoption in cost-conscious markets.
Restraint
Alternative synthesis methods competition (liquid phase synthesis, enzymatic approaches)
★★★☆☆
Alternative peptide synthesis offer different advantages and established protocols, potentially limiting solid phase resin adoption in traditional applications.
Trend
Synthesis technology integration & automation enhancement (automated synthesizers, process monitoring)
★★★★★
Advanced synthesis automation, efficiency optimization, and process analytics transform operations; technology integration and performance enhancement become core value propositions.
Trend
Customization & application-specific solutions (therapeutic targeting, specialized linkers)
★★★★☆
Custom resins for specific therapeutic applications; specialized designs and targeted synthesis capabilities drive competition toward customization solutions.
Analysis of the Solid Phase Carrier Resin for Peptide Drug Synthesis Market by Key Country
The solid phase carrier resin for peptide drug synthesis market demonstrates varied regional dynamics with Growth Leaders including China (14.3% growth rate) and India (13.3% growth rate) driving expansion through pharmaceutical development initiatives and biotechnology industry modernization. Steady Performers encompass Germany (12.2% growth rate), Brazil (11.1% growth rate), and developed regions, benefiting from established pharmaceutical industries and therapeutic peptide adoption. Mature Markets feature United States (10.1% growth rate), United Kingdom (9.0% growth rate), and Japan (8.0% growth rate), where pharmaceutical advancement and regulatory standardization requirements support consistent growth patterns.
Regional synthesis reveals East Asian markets leading adoption through pharmaceutical expansion and biotechnology development, while North American countries maintain steady expansion supported by resin technology advancement and therapeutic standardization requirements. European markets show strong growth driven by pharmaceutical applications and quality integration trends.
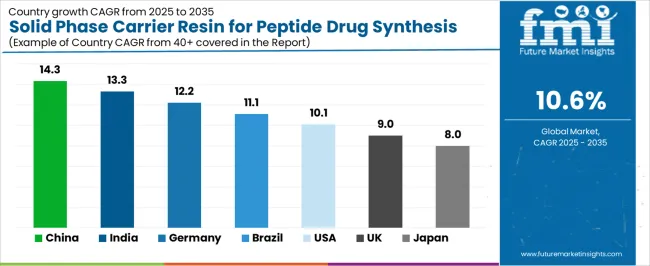
Region/Country
2025-2035 Growth
How to win
What to watch out
China
14.3%
Focus on pharmaceutical manufacturing solutions
Regulatory changes; quality standards
India
13.3%
Lead with cost-effective biotechnology applications
Import restrictions; technical barriers
Germany
12.2%
Provide premium pharmaceutical-grade resins
Over-regulation; lengthy approvals
Brazil
11.1%
Offer value-oriented therapeutic solutions
Currency fluctuations; import duties
United States
10.1%
Push technology integration
Compliance costs; scaling challenges
United Kingdom
9.0%
Focus on biotechnology applications
Economic impacts; development costs
Japan
8.0%
Emphasize precision manufacturing
Traditional preferences; adoption rates
China Drives Fastest Market Growth
China establishes fastest market growth through aggressive pharmaceutical development programs and comprehensive biotechnology industry expansion, integrating advanced carrier resin systems as standard components in pharmaceutical facilities and research installations. The country’s 14.3% growth rate reflects government initiatives promoting pharmaceutical infrastructure and domestic resin capabilities that mandate the use of professional synthesis systems in pharmaceutical and biotechnology facilities. Growth concentrates in major pharmaceutical hubs, including Beijing, Shanghai, and Guangzhou, where biotechnology development showcases integrated resin systems that appeal to pharmaceutical operators seeking synthesis optimization capabilities and therapeutic applications.
Chinese manufacturers are developing cost-effective resin solutions that combine domestic production advantages with advanced synthesis features, including enhanced coupling control and improved purity capabilities. Distribution channels through pharmaceutical suppliers and chemical distributors expand market access, while government support for biotechnology development supports adoption across diverse pharmaceutical and research segments.
Strategic Market Indicators:
Government biotechnology programs providing substantial funding for domestic resin technology development
Export market development for cost-effective synthesis solutions targeting emerging pharmaceutical markets
India Emerges as High-Growth Market
In Mumbai, Delhi, and Bangalore, pharmaceutical facilities and biotechnology operators are implementing professional carrier resin systems as standard equipment for peptide synthesis and therapeutic optimization applications, driven by increasing government pharmaceutical investment and biotechnology modernization programs that emphasize the importance of synthesis quality capabilities. The solid phase carrier resin for peptide drug synthesis market holds a 13.3% growth rate, supported by government pharmaceutical initiatives and biotechnology development programs that promote professional resin systems for pharmaceutical and research facilities. Indian operators are adopting resin systems that provide consistent synthesis performance and quality features, particularly appealing in urban regions where therapeutic development and operational excellence represent critical business requirements.
Market expansion benefits from growing pharmaceutical capabilities and international biotechnology partnerships that enable domestic production of professional resin systems for pharmaceutical and research applications. Technology adoption follows patterns established in pharmaceutical equipment, where reliability and performance drive procurement decisions and operational deployment.
Market Intelligence Brief:
Pharmaceutical modernization programs emphasizing resin systems for synthesis effectiveness and therapeutic excellence
Local manufacturers partnering with international providers for resin system development
Pharmaceutical facilities implementing resin systems for synthesis optimization and quality management
Germany Maintains Technology Leadership
Germany’s advanced pharmaceutical market demonstrates sophisticated carrier resin deployment with documented synthesis effectiveness in pharmaceutical applications and biotechnology facilities through integration with existing pharmaceutical systems and operational infrastructure. The country leverages engineering expertise in chemistry and quality systems integration to maintain a 12.2% growth rate. Pharmaceutical centers, including Bavaria, Baden-Württemberg, and North Rhine-Westphalia, showcase premium installations where resin systems integrate with comprehensive pharmaceutical platforms and facility management systems to optimize synthesis performance and operational effectiveness.
German manufacturers prioritize system quality and EU compliance in resin development, creating demand for premium systems with advanced features, including facility integration and pharmaceutical synthesis systems. The solid phase carrier resin for peptide drug synthesis market benefits from established pharmaceutical infrastructure and a willingness to invest in professional resin technologies that provide long-term operational benefits and compliance with international pharmaceutical standards.
Market Intelligence Brief:
Engineering focuses on EU standardization and quality compliance, driving premium segment growth
Technology collaboration between German manufacturers and international chemical companies
Professional training programs expanding resin system integration in pharmaceutical scenarios
Brazil Shows Strong Regional Leadership
Brazil’s market expansion benefits from diverse pharmaceutical demand, including biotechnology modernization in São Paulo and Rio de Janeiro, pharmaceutical facility upgrades, and government healthcare programs that increasingly incorporate professional resin solutions for therapeutic applications. The country maintains a 11.1% growth rate, driven by rising pharmaceutical activity and increasing recognition of professional resin benefits, including precise synthesis control and enhanced therapeutic effectiveness.
Market dynamics focus on cost-effective resin solutions that balance synthesis performance with affordability considerations important to Brazilian pharmaceutical operators. Growing pharmaceutical industrialization creates continued demand for modern resin systems in new biotechnology infrastructure and facility modernization projects.
Strategic Market Considerations:
Pharmaceutical and biotechnology segments leading growth with focus on synthesis optimization and therapeutic effectiveness applications
Regional pharmaceutical requirements driving a diverse product portfolio from basic resin systems to premium solutions
Import dependency challenges offset by potential local production partnerships with international manufacturers
Government healthcare initiatives beginning to influence procurement standards and operational requirements
United States Maintains Market Leadership
United States establishes market leadership through comprehensive pharmaceutical programs and advanced biotechnology infrastructure development, integrating carrier resin systems across pharmaceutical and research applications. The country’s 10.1% growth rate reflects established pharmaceutical industry relationships and mature resin technology adoption that supports widespread use of professional synthesis systems in pharmaceutical and biotechnology facilities. Growth concentrates in major pharmaceutical centers, including California, Massachusetts, and New Jersey, where resin technology showcases mature deployment that appeals to pharmaceutical operators seeking proven synthesis capabilities and operational efficiency applications.
American pharmaceutical providers leverage established distribution networks and comprehensive technical support capabilities, including synthesis programs and training support that create customer relationships and operational advantages. The solid phase carrier resin for peptide drug synthesis market benefits from mature regulatory standards and pharmaceutical requirements that mandate resin system use while supporting technology advancement and operational optimization.
Market Intelligence Brief:
Established regulatory standards providing consistent demand for resin technology advancement
Technology integration programs expanding synthesis capabilities in pharmaceutical scenarios
United Kingdom Shows Premium Integration
United Kingdom’s pharmaceutical market demonstrates integrated carrier resin deployment with documented synthesis effectiveness in pharmaceutical applications and biotechnology facilities through integration with existing pharmaceutical systems and operational infrastructure. The country maintains a 9.0% growth rate, supported by pharmaceutical excellence programs and synthesis effectiveness requirements that promote professional resin systems for pharmaceutical applications. Pharmaceutical facilities across England, Scotland, and Wales showcase systematic installations where resin systems integrate with comprehensive pharmaceutical platforms to optimize synthesis performance and operational outcomes.
UK pharmaceutical providers prioritize system reliability and industry compatibility in resin procurement, creating demand for validated systems with proven synthesis features, including quality monitoring integration and pharmaceutical synthesis systems. The solid phase carrier resin for peptide drug synthesis market benefits from established pharmaceutical infrastructure and excellence requirements that support resin technology adoption and operational effectiveness.
Market Intelligence Brief:
Pharmaceutical industry compatibility requirements driving standardized resin system adoption
Biotechnology partnerships providing synthesis validation and effectiveness data
Technology integration between UK pharmaceutical providers and international resin companies
Professional training programs expanding resin system deployment in pharmaceutical scenarios
Japan Shows Precision Market Development
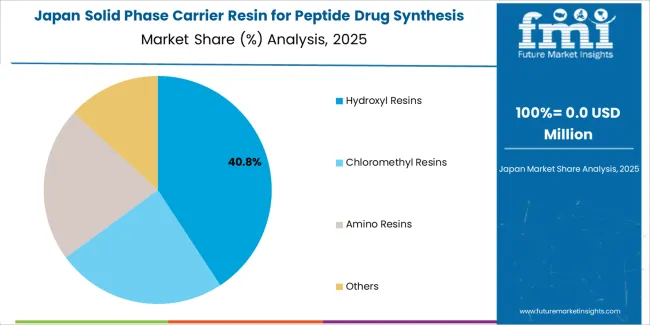
Japan’s market growth benefits from precision pharmaceutical demand, including advanced biotechnology facilities in Tokyo and Osaka, quality integration, and therapeutic enhancement programs that increasingly incorporate resin solutions for synthesis applications. The country maintains a 8.0% growth rate, driven by pharmaceutical technology advancement and increasing recognition of precision resin benefits, including accurate synthesis control and enhanced therapeutic outcomes.
Market dynamics focus on high-precision resin solutions that meet Japanese quality standards and synthesis effectiveness requirements important to pharmaceutical operators. Advanced pharmaceutical technology adoption creates continued demand for sophisticated resin systems in biotechnology facility infrastructure and therapeutic modernization projects.
Strategic Market Considerations:
Pharmaceutical and biotechnology segments leading growth with focus on precision resins and quality applications
Quality requirements driving premium product adoption from advanced resin systems
Technology integration challenges balanced by strong synthesis effectiveness and precision capabilities
Pharmaceutical quality initiatives beginning to influence procurement standards and resin requirements
Europe Market Split by Country
The European solid phase carrier resin for peptide drug synthesis market is projected to grow from USD 31.2 million in 2025 to USD 89.4 million by 2035, registering a CAGR of 11.1% over the forecast period. Germany is expected to maintain its leadership position with a 41.3% market share in 2025, supported by its advanced pharmaceutical infrastructure and major biotechnology centers.
United Kingdom follows with a 28.1% share in 2025, driven by comprehensive pharmaceutical programs and biotechnology excellence development initiatives. France holds a 16.7% share through specialized pharmaceutical applications and regulatory compliance requirements. Italy commands a 9.2% share, while Spain accounts for 4.7% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 2.9% to 3.4% by 2035, attributed to increasing pharmaceutical adoption in Nordic countries and emerging biotechnology facilities implementing pharmaceutical modernization programs.
Competitive Landscape of the Solid Phase Carrier Resin for Peptide Drug Synthesis Market
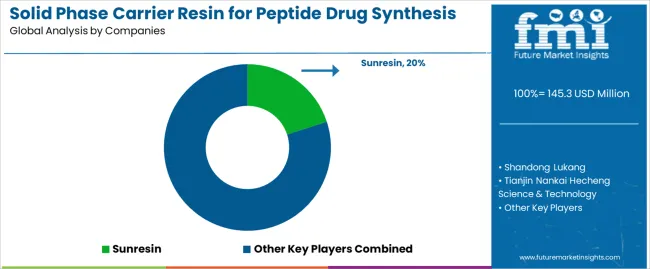
Structure: ~8-12 credible players; top 3-5 hold ~70-75% by revenue.
Leadership is maintained through: chemical expertise, pharmaceutical relationships, and product innovation (resin technology + synthesis efficiency + therapeutic applications).
What’s commoditizing: basic resin chemistry and standard synthesis systems.
Margin Opportunities: custom resin development, therapeutic specialization, and integration into pharmaceutical workflows (synthesis optimization, quality control).
Stakeholder
What they actually control
Typical strengths
Typical blind spots
Global chemical companies
Supply chain reach, broad product portfolios, pharmaceutical relationships
Wide availability, proven quality, multi-region support
Product innovation cycles; customer dependency on pharmaceutical validation
Technology innovators
Resin R&D; advanced synthesis technologies; enhanced performance properties
Latest technologies first; attractive ROI on synthesis effectiveness
Service density outside core regions; scaling complexity
Regional specialists
Local compliance, fast delivery, nearby customer support
“Close to customer” support; pragmatic pricing; local regulations
Technology gaps; talent retention in customer service
Full-service providers
Synthesis programs, technical services, method development
Lowest operational risk; comprehensive support
Service costs if overpromised; technology obsolescence
Niche specialists
Specialized applications, custom resins, therapeutic services
Win premium applications; flexible configurations
Scalability limitations; narrow market focus
Key Players in the Solid Phase Carrier Resin for Peptide Drug Synthesis Market
Sunresin
Shandong Lukang
Tianjin Nankai Hecheng Science & Technology
Zhejiang Zhengguang
Jiangsu Haipu Functional Materials
RawPEG
Merck
CEM Corporation
ChemPep Inc.
Iris Biotech
Bachem
Gyros Protein Technologies
AAPPTec
Advanced ChemTech
Peptides International
GL Biochem
Chempep
Scope of the Report
Item
Value
Quantitative Units
USD 145.3 million
Resin Type
Hydroxyl Resins, Chloromethyl Resins, Amino Resins, Others
Application
Orthopedics, Cardiovascular, Metabolic Diseases, Tumors, Others
End Use
Pharmaceutical Companies, Biotechnology Firms, Contract Research Organizations, Academic Institutions, Specialty Chemical Manufacturers
Regions Covered
North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, Middle East & Africa
Countries Covered
China, India, Germany, Brazil, United States, United Kingdom, Japan, Canada, France, Australia, and 25+ additional countries
Key Companies Profiled
Sunresin, Shandong Lukang, Tianjin Nankai Hecheng Science & Technology, Zhejiang Zhengguang, Jiangsu Haipu Functional Materials
Additional Attributes
Dollar sales by resin type and application categories, regional adoption trends across East Asia, North America, and Western Europe, competitive landscape with chemical manufacturers and pharmaceutical suppliers, pharmaceutical operator preferences for synthesis effectiveness and therapeutic optimization, integration with pharmaceutical platforms and quality management systems, innovations in resin technology and synthesis enhancement, and development of advanced carrier resin solutions with enhanced performance and pharmaceutical optimization capabilities.
Solid Phase Carrier Resin for Peptide Drug Synthesis Market by Segments Resin Type:
Hydroxyl Resins
Chloromethyl Resins
Amino Resins
Others
Application:
Orthopedics
Cardiovascular
Metabolic Diseases
Tumors
Others
End Use:
Pharmaceutical Companies
Biotechnology Firms
Contract Research Organizations
Academic Institutions
Specialty Chemical Manufacturers
Region:
North America
United States
Canada
Mexico
Latin America
Brazil
Chile
Rest of Latin America
Western Europe
Germany
United Kingdom
France
Italy
Spain
Nordic
BENELUX
Rest of Western Europe
Eastern Europe
Russia
Poland
Rest of Eastern Europe
East Asia
South Asia Pacific
India
ASEAN
Australia & New Zealand
Rest of South Asia Pacific
Middle East & Africa
Kingdom of Saudi Arabia
Other GCC Countries
Turkey
South Africa
Other African Union
Rest of Middle East & Africa
Frequently Asked Questions
How big is the solid phase carrier resin for peptide drug synthesis market in 2025?
The global solid phase carrier resin for peptide drug synthesis market is estimated to be valued at USD 145.3 million in 2025.
What will be the size of solid phase carrier resin for peptide drug synthesis market in 2035?
The market size for the solid phase carrier resin for peptide drug synthesis market is projected to reach USD 397.8 million by 2035.
How much will be the solid phase carrier resin for peptide drug synthesis market growth between 2025 and 2035?
The solid phase carrier resin for peptide drug synthesis market is expected to grow at a 10.6% CAGR between 2025 and 2035.
What are the key product types in the solid phase carrier resin for peptide drug synthesis market?
The key product types in solid phase carrier resin for peptide drug synthesis market are hydroxyl resins, chloromethyl resins, amino resins and others.
Which application segment to contribute significant share in the solid phase carrier resin for peptide drug synthesis market in 2025?
In terms of application, orthopedics segment to command 14.0% share in the solid phase carrier resin for peptide drug synthesis market in 2025.

