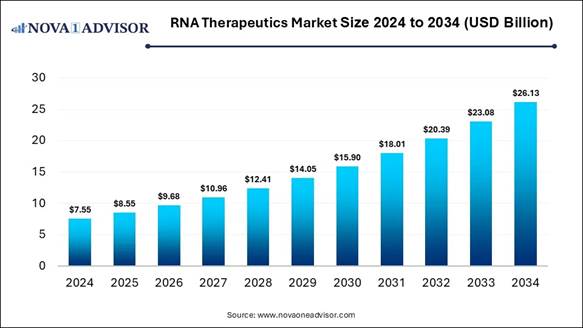
According to Nova One Advisor, the global RNA
therapeutics market size is expected to be worth around 26.13 billion
by 2034, increasing from USD 8.55 billion in 2025, representing a healthy CAGR
of 13.22% from 2025 to 2034.
The RNA therapeutics market is growing as
it is a significant tool for the treatment of various types of serious
diseases, particularly genetically mutated diseases such as cancer, metabolic
disorders. With the growing applications of genome sequencing, growing numbers
of intronic variants will be recognized by clinical diagnostic testing. RNA
analysis provides insight into the diseases and mechanisms leading to death and
has developed into a valuable technology for the diagnosis of the cause of
death in forensic science.
The Complete Study is Now Available for
Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/9205
RNA Therapeutics Market Highlights:
⬥︎ North America dominated the RNA
therapeutics market with the largest share in 2024.
⬥︎ Asia Pacific is expected to grow at
the fastest CAGR during the forecast period of 2025 and 2034.
⬥︎ By type, the mRNA therapeutics segment
led the market in 2024.
⬥︎ By type, the RNA interference (RNAi)
therapeutics segment is expected to expand at the highest CAGR in the coming
years.
⬥︎ By product, the vaccines segment held
the largest share of the market in 2024.
⬥︎ By product, the drugs segment is
likely to grow at a significant rate over the forecast period.
⬥︎ By indication, the infectious diseases
segment dominated the market in 2024.
⬥︎ By indication, the rare genetic
disorders/hereditary diseases segment is expected to expand at a rapid pace in
the upcoming period.
⬥︎ By end-user, the hospitals &
clinics segment held the largest market share in 2024.
⬥︎ By end-user, the research institutes
segment is expected to grow at the fastest rate during the projection period.
Market Overview and Industry Potential
Ribonucleic acid (RNA)-based
therapies are an emergent area of therapeutic development that provides the
strength to produce novel treatments for a wide range of conditions. These
therapeutics are utilized to characterize mRNA expression profiles in different
tissues and progress assays for particular mRNA molecules in forensic contexts.
RNA
sequencing has become a model technology in modern biology and clinical
science. Its enormous popularity is due in large part to the incessant efforts
of the bioinformatics community to progress precise and scalable computational technologies
to analyze the massive amounts of transcriptomic data that it produces.
RNA research has gathered noteworthy
attention due to its profound consequences in drug development and therapeutic
discovery. Advanced research in RNA biology integrated with progress in
delivery technologies holds the promise of renovating RNA-based therapeutics
into conventional clinical practice. RNA-Seq identifies differentially
expressed transcripts and reveals novel molecular mechanisms of disease.
Approved RNA Therapies as of Q3 2024
Product Name
Generic Name
Year
First Approved
Disease(s)
Locations Approved
Originator Company
CSPC Pharmaceutical COVID-19 vaccine
COVID-19 vaccine, CSPC Pharmaceutical
2023
Infection, coronavirus, novel
coronavirus prophylaxis
China
CSPC Pharmaceutical
Sinocelltech COVID-19 vaccine
COVID-19 alpha/beta/delta/Omicron variants
S-trimer quadrivalent recombinant protein vaccine
2023
Infection, coronavirus, novel
coronavirus prophylaxis
China, UAE, U.S.
Sinocelltech
Izervay
avacincaptad pegol sodium
2023
Wet age-related macular
degeneration
U.S.
Archemix
Qalsody
tofersen
2023
Amyotrophic lateral sclerosis
U.S., EU
Ionis Pharmaceuticals
ARCT-154
COVID-19 mRNA vaccine, Arcturus
2023
Infection, coronavirus, novel
coronavirus prophylaxis
Japan
Arcturus Therapeutics
Daichirona
COVID-19 vaccine, Daiichi Sankyo
2023
Infection, coronavirus, novel
coronavirus prophylaxis J
Japan
Daiichi Sankyo
Wainua
eplontersen
2023
Transthyretin-related hereditary
amyloidosis
U.S., Canada
Ionis Pharmaceuticals
Rivfloza
nedosiran
2023
Hyperoxaluria
U.S.
Dicerna Pharmaceuticals
SYS-6006.32
Bivalent COVID-19 mRNA
vaccine, CSPC Pharmaceutical
2023
Infection, coronavirus, novel coronavirus
prophylaxis
China
CSPC Pharmaceutical
RQ-3033
COVID-19 mRNA vaccine,
Walvax Biotechnology
2023
Infection, coronavirus, novel
coronavirus prophylaxis
China
Walvax Biotechnology
Rytelo
imetelstat
2024
Myelodysplastic syndrome
U.S.
Geron
mRESVIA
respiratory syncytial virus
vaccine, Moderna
Therapeutics
2024
Respiratory syncytial virus
prophylaxis
U.S., EU
Moderna Therapeutics
Latest Trends of the Market
✔
In August 2025, Alnylam
Pharmaceuticals, Inc., the leading RNAi therapeutics company, announced
that the Company will present new data from its hypertension and transthyretin
amyloidosis (ATTR) programs at the upcoming European Society of Cardiology
(ESC) Congress
✔
In May 2025, Biogen Inc. and City Therapeutics, Inc., a
privately held biopharmaceutical company leading the future of RNA interference
(RNAi)-based medicine, announced a strategic collaboration to develop select
novel RNAi therapies. Through the collaboration, City Therapeutics will
leverage its next-generation RNAi engineering technologies to develop an RNAi
trigger molecule combined with proprietary drug delivery technology from
Biogen.
Recent Advancements in RNA Sequencing:
Market’s Largest Potential
Recent developments in high-throughput RNA
sequencing, single-cell RNA sequencing, and epitranscriptomics further
unravelled the complexity of RNA biology, shedding light on the details of gene
regulation and the diversity of cells. The incorporation of computational
devices and bioinformatics has driven the identification of RNA-driven
biomarkers and the development of RNA
therapeutics.
⬥︎ For Instance, In June 2025, BioNTech
SE and CureVac N.V. announced that they had entered into a definitive Purchase
Agreement pursuant to which BioNTech intended to acquire all of the shares of
CureVac, a clinical-stage biotech company developing a novel class of
transformative medicines in oncology and infectious diseases based on messenger
ribonucleic acid.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/9205
Report Scope of RNA Therapeutics Market
Report Coverage
Details
Market Size in 2025
USD 8.55 Billion
Market Size by 2034
USD 26.13 Billion
Growth Rate From 2025 to 2034
CAGR of 13.22%
Base Year
2024
Forecast Period
2025-2034
Segments Covered
By Type, By Product, By Indication, By
End-User, By Region
Market Analysis (Terms Used)
Value (US$ Million/Billion) or
(Volume/Units)
Regional scope
North America; Europe; Asia Pacific;
Latin America; MEA
RNA Therapeutics Market Segmentation
Analysis:
By Type Analysis:
The mRNA
therapeutics segment dominated in the RNA therapeutics market, as it is
used for a variety of diseases that are resistant to present treatments, like
metabolic genetic diseases, infectious diseases, cancer, cardiovascular
disease, cerebrovascular diseases, and others. mRNA-based therapeutics are
characterized by their fast advancement and manufacturing capability. mRNA
technology permits the quick testing of hundreds of potential vaccine targets.
On the other hand, the RNA interference
(RNAi) therapeutics segment is expected to grow significantly during the
forecast period, as it can target potentially any gene in the genome, containing
targets that are undruggable by small molecules and antibodies. They have more
potent and durable effects. It is administered by multiple routes, intravenous
(IV), subcutaneous, and intrathecal delivery.
By Product Analysis:
The vaccines
segment dominated the market in 2024, as it has high efficiency in preventing
serious diseases. The significant advantages of mRNA
vaccines are their higher success rate in preventing severe
hospitalization, illness, and death. RNA vaccines have benefits in safety,
efficacy, affordability, speed, and simplicity of production.
On the other hand, the drugs segment is
expected to grow at the fastest CAGR in the market during the forecast period,
as RNA-based drugs are a quickly expanding category of drugs based on RNA
molecules goal of treating or preventing diseases.
By Indication Analysis:
The infectious
diseases segment dominated the market in 2024, as mRNA vaccines have
the potential for the fastest response to large-scale outbreaks of infectious
diseases, like COVID-19. It has always been the researchers’ intention to
enhance the stability, translation efficiency, immunogenicity, and delivery
system to achieve effective and harmless delivery of mRNA for treating
infectious diseases.
On the other hand, the rare genetic
disorders/hereditary diseases segment is expected to grow at the fastest CAGR
in the market during the forecast period, as mRNA therapies for rare genetic
diseases, like protection against degradation and immune clearance, organ
targeting, and lowering intracellular immunotoxicity, along with delivery
vehicles and mRNA modifications for efficient expression and lifespan. mRNA
treatments to address the large unmet requirement in rare genetic disorders.
By End User Analysis:
The hospitals and clinics segment dominated
the market in 2024, as their strength to target undruggable targets that
traditional therapeutics cannot. RNA therapeutics, predominantly ASOs and
siRNAs, bind to their target through sequence‐specific
binding. Novel RNA therapeutics are rapidly developed and produced using
current modification processes and delivery technologies.
On the other hand, the research institutes
segment is expected to grow at the fastest CAGR in the market during the
forecast period, as RNA-based therapies show a promising and fast advancing
sector in medicine. The unique characteristics of RNA, its ability to regulate
gene expression, its flexibility in targeting specific genes, and its potential
for targeted medicine have created the opportunity for the development of novel
treatments. RNA-driven therapeutics hold huge potential for managing a broad
range of diseases, including infectious diseases, cancer, and genetic
disorders.
Regional Insights
North America led the RNA therapeutics
market in 2024, as the growing prevalence of chronic and rare disease
conditions, combined with a vital requirement for precision medicine, is
driving investments and progress in the RNA solution space. Increasing
investments in RNA science, increasing awareness of genetic disorders, and the
successful deployment of mRNA-based vaccines.
⬥︎ For Instance, In September 2025,
Illumina Inc. announced the launch of Illumina Protein Prep, an assay
introducing superior performance for next-generation sequencing (NGS) based
proteomics discovery at scale. Illumina Protein Prep has been available through
an early-access program and is broadly available to customers worldwide,
enabling researchers to add proteomics to large-scale genomics studies and
drive new insights across cancer and cardio metabolic, and immunologic
diseases, with a streamlined sample-to-insights solution for discovery and
clinical research.
In the U.S., noteworthy government and
private spending in research and development, an advanced biotechnology sector,
and presence skilled workforce, helpful regulatory pathways from the US FDA,
the potential to manage genetic disorders, and development in delivery systems,
driving the growth of the market.
Why is Asia Pacific the Fastest Growing
in the RNA Therapeutics Market?
APAC is fastest fastest-growing region in
the market, with revolutionary technological advancements, growing healthcare
applications, and a speedily growing pipeline of novel therapeutics. Recent
developments in RNA delivery, modification, and manufacturing are driving wider
acceptance and commercialization, enhancing the precision of RNA treatments and
creating opportunities for targeted medicine and gene therapies.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/9205
Region-Wise Growth Overview of the RNA
Therapeutics Market:
Region
Market Size (2024)
Projected CAGR (2025-2034)
Key Growth Drives
Key Challenges
Market Outlook
North America
USD 3.1 Bn
~6.65%
Advanced R&D capabilities, robust
biotech infrastructure, strong funding support
High development costs, stringent
regulatory requirements
Dominant and steadily growing market
Asia Pacific
USD 2.2 Bn
~7.89%
Rising healthcare investments, large
patient base, supportive government policies
Infrastructure gaps, price sensitivity in
some markets
Fastest-growing and rapidly emerging
region
Europe
USD 1.8 Bn
~10.76%
Healthcare innovation, collaborative
R&D, regulatory facilitation (e.g., EMA)
Slow regulatory pathways, reimbursement
constraints
Stable growth
Latin America
USD 0.6 Bn
~5.24%
Expanding healthcare infrastructure,
growing demand for advanced therapies
Limited funding, regulatory complexity
Emerging market with strong growth
potential
MEA
USD 0.4 Bn
~4.14%
Government-backed healthcare projects,
increasing biotech interest
Infrastructure deficiencies, skilled
workforce shortages
Underpenetrated but promising growth
RNA Therapeutics Market Companies:
• ISIS Pharmaceuticals
• Quark Pharmaceuticals
• Alnylam Pharmaceuticals
• Dice a Pharmaceuticals
• Tekmira Pharmaceuticals Corp.
• Benitec Biopharma Limited
• Genzyme Corporation
• Silence Therapeutics PLC
• Cenix BioScience GmbH
What is Going Around the Globe?
⬥︎ In March 2025, Alnylam
Pharmaceuticals, Inc., the leading RNAi therapeutics company, highlighted the
significance of the U.S. Food and Drug Administration’s approval of Qfitlia,
the sixth Alnylam-discovered RNAi therapeutic approved in the U.S., and the first
and only therapeutic to lower antithrombin (AT), a protein that inhibits blood
clotting, to promote thrombin generation to rebalance hemostasis and prevent
bleeds.
⬥︎ In June 2025, Lexeo Therapeutics,
Inc., a clinical-stage genetic medicine company dedicated to pioneering novel
treatments for cardiovascular diseases, announced a strategic partnership to
develop therapies for genetic cardiac diseases utilizing a novel non-viral RNA
platform. Combined with investment of up to $40 million from leading life-sciences
investors Perceptive Xontogeny Venture Funds and venBio Partners, the
partnership seeks to further revolutionize the treatment of cardiovascular
diseases.
⬥︎ In January 2025, Inverna Therapeutics,
a novel Danish biotech company at the forefront of innovative RNA therapeutics,
announced its launch as a leading-edge RNA therapeutics company. The Company
was co-founded by the University of Southern Denmark and Argobio and is
dedicated to transforming patient outcomes in severe genetic diseases, starting
with a lead program addressing Huntington’s disease.
You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344
Related Report
⬥︎ mRNA Therapeutics Market – https://www.precedenceresearch.com/mrna-therapeutics-market
⬥︎ U.S. mRNA Therapeutics Market – https://www.precedenceresearch.com/us-mrna-therapeutics-market
⬥︎ Next-Generation RNA Therapeutics Market – https://www.precedenceresearch.com/next-generation-rna-therapeutics-market
⬥︎ mRNA Therapeutics CDMO Market – https://www.precedenceresearch.com/mrna-therapeutics-cdmo-market
Segments Covered in the Report
This report forecasts revenue growth at
country levels and provides an analysis of the latest industry trends in each
of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc.
has segmented the RNA therapeutics market.
By Type
• RNA Interference (RNAi) Therapeutics
• mRNA Therapeutics
• Antisense Oligonucleotide (ASO)
Therapeutics
• Others
By Product
• Vaccines
• Drugs
By Indication
• Rare Genetic Disorders/Hereditary
Diseases
• Infectious Diseases
• Others (Metabolic Disorders, Auto-immune
Disorders, and Oncology)
By End-User
• Research Institutes
• Hospitals & Clinics
By Regional
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East and Africa (MEA)
Immediate Delivery Available | Buy This
Premium Research https://www.novaoneadvisor.com/report/checkout/9205
About-Us
Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep,
data-driven insights that power innovation and transformation across
industries. With a sharp focus on the evolving landscape of life sciences, we
specialize in navigating the complexities of cell and gene therapy, drug
development, and the oncology market, enabling our clients to lead in some of
the most revolutionary and high-impact areas of healthcare.
Our expertise spans the entire
biotech and pharmaceutical value chain, empowering startups, global
enterprises, investors, and research institutions that are pioneering the next
generation of therapies in regenerative medicine, oncology, and precision
medicine.
Web: https://www.novaoneadvisor.com/
Contact Us
USA: +1 804 420 9370
Email: sales@novaoneadvisor.com
For Latest Update Follow Us: LinkedIn

