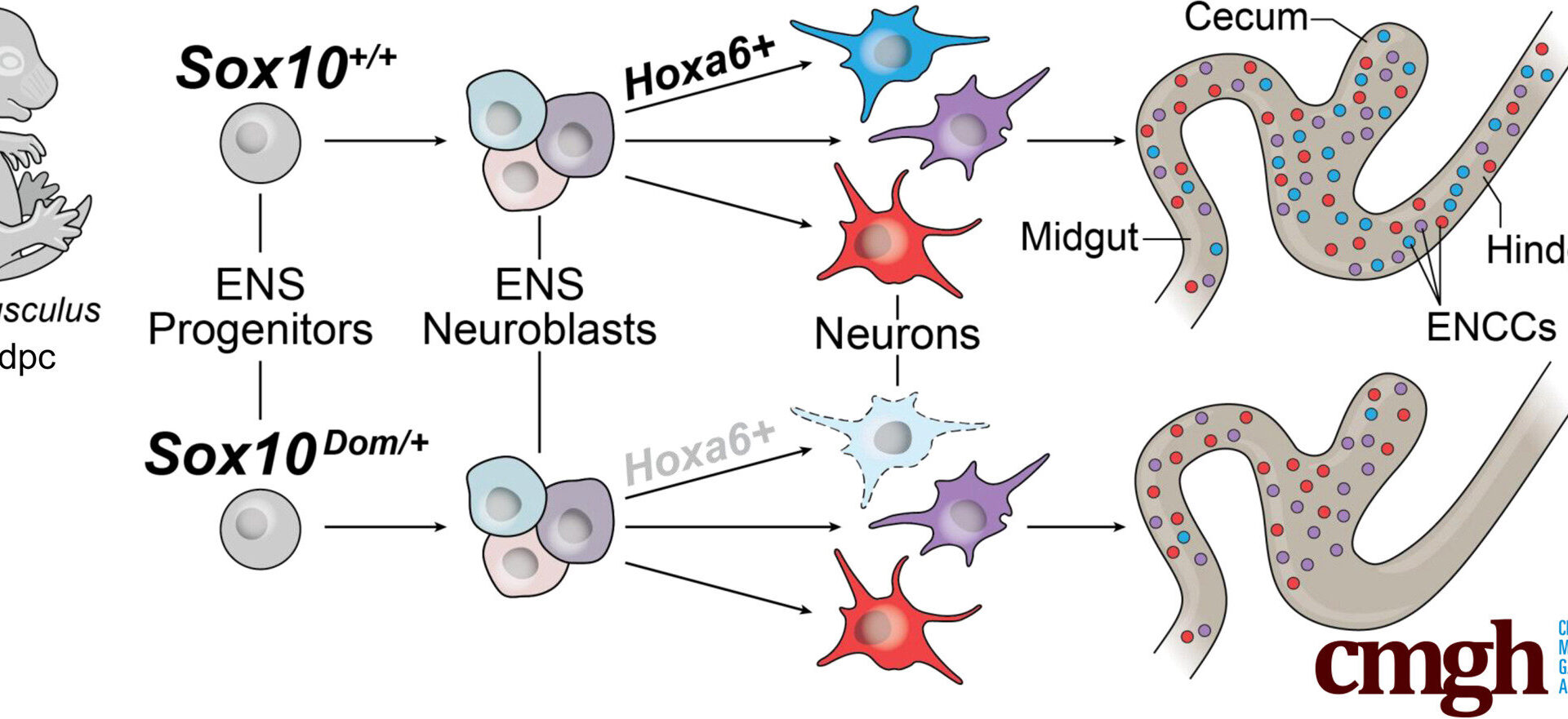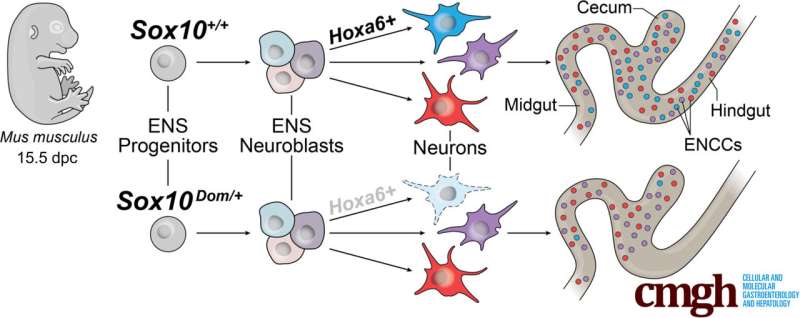
Graphical abstract. Credit: Cellular and Molecular Gastroenterology and Hepatology (2025). DOI: 10.1016/j.jcmgh.2025.101590
Vanderbilt researchers, including those from the Vanderbilt Brain Institute, have made significant strides in understanding how the enteric nervous system—sometimes called the “brain” of the gut—forms and functions.
In a study published in Cellular and Molecular Gastroenterology and Hepatology, the lab of principal investigator, Michelle Southard-Smith, sheds light on how the SOX10 protein contributes to the development of gut cells that play a role in gastrointestinal motility, or how food moves through the digestive system.
The paper is titled “Single Cell Profiling in the Sox10Dom Hirschsprung Mouse Implicates Hox genes in Enteric Neuron Trajectory Allocation.”
The new research offers insights that may advance treatments for gastrointestinal motility ailments like Hirschsprung disease (a birth condition that prevents normal movement of stool through the colon), irritable bowel syndrome, and chronic constipation.
Specialized nerve cells in the gut, known as enteric neurons, are vital for moving food through the digestive system and maintaining overall health. When these neurons don’t develop or function properly, the coordinated muscle contractions that push food along can become irregular or weak.
This disruption can lead to motility conditions, where food may move too slowly, too quickly, or in an uncoordinated way—causing symptoms like abdominal pain, constipation, or diarrhea.
To guide treatment development for gut motility disorders, scientists need to understand which genes and signals guide their formation. However, according to Southard-Smith, a professor of cell and developmental biology and medicine, “very little is understood” about the gene regulatory networks that control the formation of diverse types of enteric neurons .
“We need to know which genes and signaling pathways are needed for this process,” she said.
In her latest study, members of the Southard-Smith lab, including first authors Justin Avila and Joseph Benthal, used a combination of genetically engineered mouse models and high-resolution, single-cell RNA sequencing to probe the earliest stages of neuron formation in the enteric nervous system.
The researchers’ deep sequencing strategy went well beyond standard approaches, allowing the team to capture transient transcription factors that often go undetected. Transcription factors are proteins that regulate the expression of different genes: They decide if DNA is made into RNA, which is subsequently then made into protein.
“We sequenced our samples much more deeply than is typical. Unexpectedly, this deep sequencing led to identification of greater diversity among developing enteric neurons, which emphasizes just how important those early lineage choices are,” Southard-Smith said. “Justin and Joseph worked together as a team to derive these new insights for the ENS field.”
Among the study’s top findings
The SOX10 protein is present in early-forming enteric neurons, the specialized nerve cells in the gut that control digestion. This finding is contrary to previous beliefs that SOX10—a gene-regulating protein known for helping guide the development of the ENS—is restricted to progenitor and glial cells.
Progenitor cells are early-stage cells that can develop into nerve or support cells, while glial cells are the support team that keep nerve cells in the gut healthy and functioning. This research found the SOX10 protein within early enteric neurons themselves.
Mutations in the Sox10 gene cause early developmental shifts. When the Sox10 gene is altered, the earliest stages of nerve cell development change course. These changes disrupt neuronal trajectories—the step-by-step developmental “paths” that immature nerve cells follow to become specific, fully functional neuron types.
A mutation shift at this early stage can ripple forward, resulting in the wrong balance of neuron types in the gut, which persist even after birth and may impair the gut’s ability to move food normally.
The Hox gene family plays a bigger role in gut development than expected. Hox genes are a group of “master” regulatory genes that act like a body blueprint, telling cells where they are in the body and what they should become. This study found that many Hox genes in the developing gut are active in early enteric neurons, including the gene Hoxa6, which had never been detected in the ENS before.
When Sox10 was mutated, Hoxa6 activity dropped, and the neurons that normally appear in the developmental path where Hoxa6 is usually active were missing—linking Hoxa6’s activity directly to the presence or absence of certain neuron types in the gut.
In the short term, the team plans to determine which of the Hox genes that were detected for the first time in the ENS are directly regulated by the SOX10 transcription factor. They also aim to better map the gene regulatory networks governing ENS development.
The ultimate goal, however, is therapeutic application. “We expect that the genes we’ve uncovered through these deep sequencing studies of early neuronal progenitors will be key factors that must be switched on to form specific types of neurons for transplantation into the GI tracts of patients with Hirschsprung disease or other motility disorders,” Southard-Smith said.
Such transplants could restore the missing or malfunctioning nerve cells that control muscle contractions in the gut, helping to re-establish normal movement of food and waste through the digestive system.
The study relied heavily on Vanderbilt and Vanderbilt University Medical Center core facilities, including the Cell Imaging Shared Resource and the Flow Cytometry Shared Resource, and staff members provided crucial technical expertise.
More information:
Justin A. Avila et al, Single Cell Profiling in the Sox10 Hirschsprung Mouse Implicates Hox Genes in Enteric Neuron Trajectory Allocation, Cellular and Molecular Gastroenterology and Hepatology (2025). DOI: 10.1016/j.jcmgh.2025.101590. www.sciencedirect.com/science/ … ii/S2352345X25001316
Provided by
Vanderbilt University
Citation:
Researchers uncover critical genetic drivers of the gut’s ‘nervous system’ development (2025, September 5)
retrieved 6 September 2025
from https://medicalxpress.com/news/2025-09-uncover-critical-genetic-drivers-gut.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.