Preeclampsia Market Summary
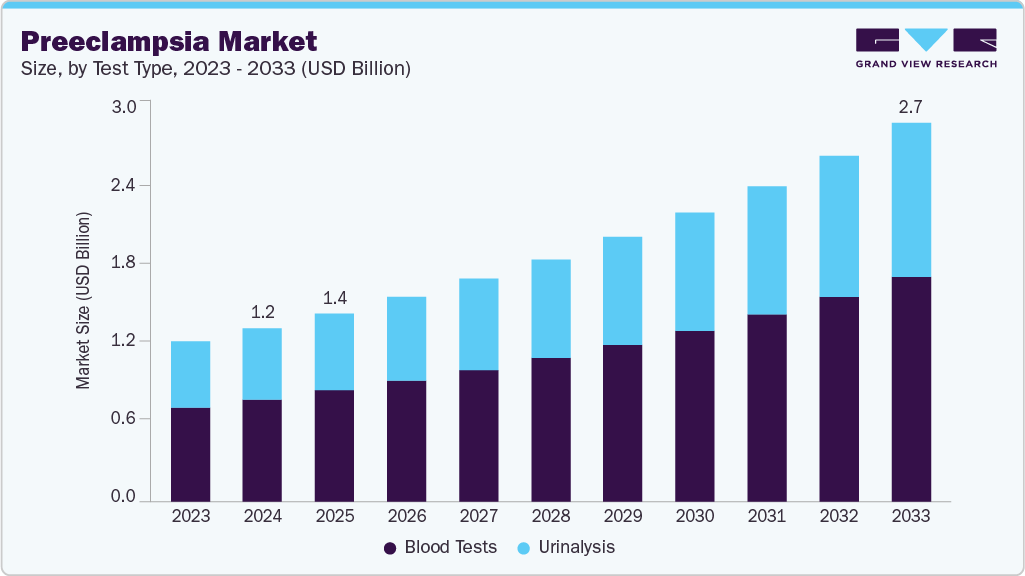
The global preeclampsia market size was valued at USD 1.25 billion in 2024 and is expected to reach USD 2.73 billion by 2033, growing at a CAGR of 9.12% from 2025 to 2033. The market is driven by rising incidences of the preeclampsia and the rising demand for early and accurate diagnostic tests to predict and manage risks before symptoms grow.
Key Market Trends & Insights
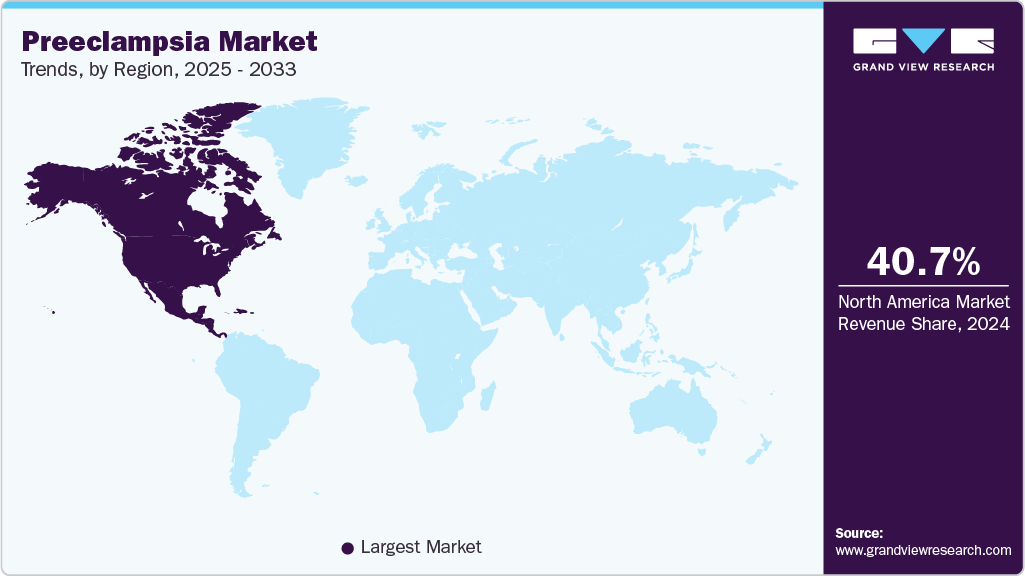
North America preeclampsia market dominated the global market and accounted for the largest revenue share of 40.66% in 2024.
The U.S. led the North American market and held the largest revenue share in 2024
Based on test type, the blood test segment dominated the global market and accounted for the largest revenue share of 58.97% in 2024.
Based on application, the universal screening segment held the largest revenue share of 40.31% in 2024.
Based on end use, the diagnostics centers segment held the largest revenue share of 35.25% in 2024.
Market Size & Forecast
2024 Market Size: USD 1.25 Billion
2033 Projected Market Size: USD 2.73 Billion
CAGR (2025-2033): 9.12%
North America: Largest market in 2024
Globally, there are near about 140 million births annually; in the U.S. alone, about 3.7 million births per year, of which up to 30 % of pregnancies are affected by complications (including preterm birth, preeclampsia, restricted growth, etc.). In April 2025, Mirvie highlighted that its simple blood test can predict preeclampsia risk in pregnancy as early as 17.5 to 22 weeks-helping clinicians stratify patients and intervene sooner. Preeclampsia is a hypertensive disorder of pregnancy that affects roughly 2 % to 8 % of pregnancies worldwide. It typically presents after 20 weeks of gestation, and is diagnosed by elevated blood pressure and signs of organ dysfunction such as proteinuria. If undetected or unmanaged, the condition can progress to eclampsia (seizures), HELLP syndrome, placental abruption, or other severe complications. Each year, preeclampsia is estimated to be responsible for about 46,000 maternal deaths and 500,000 fetal or newborn deaths globally. Because it contributes significantly to maternal and perinatal morbidity, there is strong demand for better preventive, diagnostic, and management tools.
Preeclampsia is a serious blood pressure condition in the U.S. that affects about 1 in 12 to 1 in 25 pregnancies and is on the rise. After 20 weeks of pregnancy, high blood pressure and indicators of organ damage, such as protein in the urine, which suggests kidney damage, are necessary for the diagnosis. A history of preeclampsia, pre-existing chronic diabetes or hypertension, and first-time pregnancy are risk factors.
In the recent year’s companies such as PerkinElmer added DELFIA Xpress sFlt-1 kit to its CE-IVD pre-eclampsia product offerings. The Thermo Fisher BRAHMS biomarker assays (sFlt-1 / PlGF) are now marketed for preeclampsia management in labs, offering precise risk calculations. As new molecular tests, such as cell-free RNA assays (Mirvie) or predictive risk scores, mature and get regulatory approval, they will broaden the market’s reach into earlier prenatal screening. The global market’s growth is further supported by rising maternal health focus, growing awareness in emerging economies, and increased investment in women’s health technologies.
Adoption is uneven, though. Despite their great need, advanced tests are not widely adopted in low-resource settings due to a lack of infrastructure for biomarker assays, financial limitations, and gaps in prenatal care. The WHO notes that even with guidelines, implementation lags and magnesium sulfate is still underutilized in many low-resource settings. Furthermore, in many areas, market penetration for novel diagnostics is slowed by regulatory and reimbursement barriers. However, these obstacles might lessen as costs come down and test formats (like point-of-care or home sampling) get easier.
Company Case study ( Sera Prognostics )
As of October 2025, Sera positions itself as a leader in pregnancy biomarker testing, aiming to redefine prenatal care by identifying risks early (especially for preterm birth).
Their flagship product is the PreTRM test, a blood-based biomarker assay administered between weeks 18–20 of gestation in singleton pregnancies. This test forecasts risk for spontaneous preterm birth, enabling early intervention strategies.
They emphasize combining their proprietary proteomics, immunoassay development, and bioinformatics / algorithmic analyses to build differentiated diagnostic offerings.
A major selling point is economic impact: by detecting risk earlier, they claim that targeted interventions may reduce costly neonatal complications and overall health care costs.
Sera claims that using PreTRM plus targeted management strategies demonstrated an 18% reduction in severe neonatal morbidity and mortality in a published AVERT trial (date in their website).
The PRIME study results were slated for presentation at the 2025 SMFM (Society for Maternal-Fetal Medicine) meeting.
Their pipeline includes expanding from mass-spectrometry discovery to immunoassays for more practical tests, plus potential new biomarkers and predictive models built on their proprietary biobank.
Market Concentration & Characteristics
The market for preeclampsia is known for its steady pace of innovation. For example, in May 2023, Thermo Fisher received FDA clearance for two immunoassays (PlGF plus and sFlt-1) that help assess a pregnant woman’s risk of progressing to severe preeclampsia within two weeks. These innovations push the market toward more personalized prenatal care, with better risk stratification, and thus act as strong impetus for investment, adoption, and expansion in this sector.
Mergers and acquisitions in this space are driven by the company’s expanding their technological capabilities and service portfolios. In September 2024, Trinity Biotech announced the acquisition of Metabolomics Diagnostics (developer of the PrePsia early-pregnancy predictive test) for a modest enterprise value, acquiring mass-spectrometry and machine-learning capabilities to commercialize PrePsia (first-trimester risk prediction for preeclampsia) via Trinity’s Immco reference lab in the U.S. and to pursue international rollout, with the company stating first revenues from the test were expected in 2025.
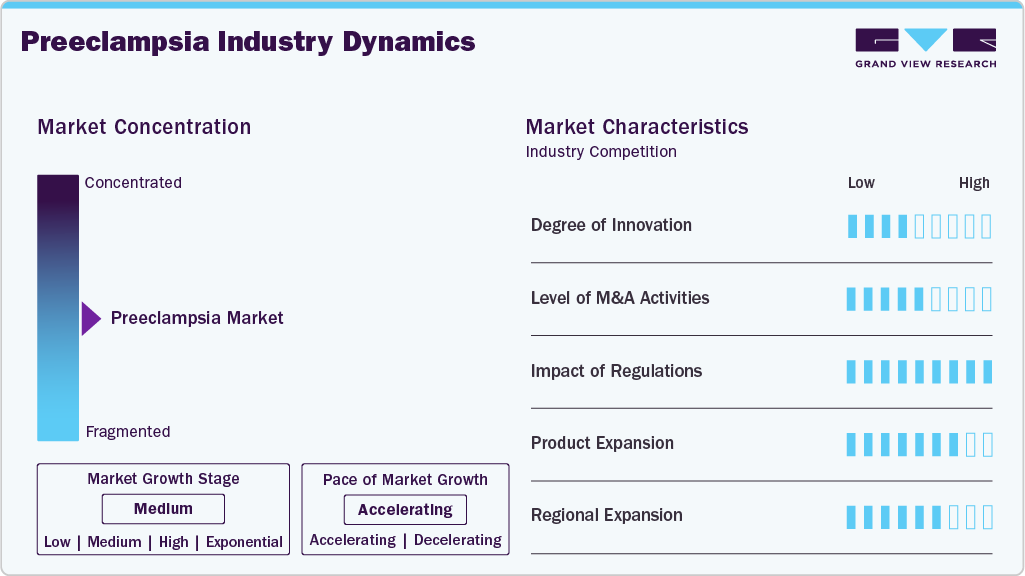
Regulatory frameworks play a critical role in shaping market growth, especially in countries like U.S, UK, India. The Thermo Fisher assays were classified via De Novo FDA classification (Class II) in May 2023, establishing special controls for prognostic tests for progression of preeclampsia. FDA Access Data U.S. Food and Drug Administration. This precedent sets a regulatory pathway for future biomarker tests in this niche, but also underscores how regulatory classification and clinical evidence requirements remain critical gating factors for new entrants.
Product expansion remains a key strategy for growth. Diagnostic service providers are expanding beyond single assays into service models and clinical test offerings. For example, Trinity Biotech is launching its reference-lab offering of the PreClara ratio test to broaden access to high-performance biomarker diagnostics. Such moves reflect the trend of firms diversifying beyond just instrument or reagent sales into service and access models to drive adoption.
Countries with increasing awareness of maternal health complications, especially in developing regions, are witnessing higher adoption of preeclampsia diagnostics and treatments. Collaborating with local distributors or hospitals to penetrate new regions efficiently. In July 2025, WIN expands maternal health program in U.S. and introduces early preeclampsia prediction and prevention program powered by Mirvie’s Encompass WIN (the fertility and maternity benefits provider) has expanded its WINMaternity service offering by integrating Mirvie’s Encompass, a predictive blood test solution for early preeclampsia risk assessment.
Test Type Insights
On the basis of test type, blood tests segment accounted for the largest market share of 58.97% in 2024. The primary driver of this growth is the advancements in identifying specific biomarkers associated with preeclampsia. The use of blood-based diagnostic techniques has been further accelerated by the rising incidence of hypertensive disorders during pregnancy. Blood tests are now a preferred option for the early detection and monitoring of preeclampsia due to improvements in accuracy and reliability brought about by biomarker discovery innovations and the integration of cutting-edge diagnostic technologies. In February 2025, a new blood test that has an 80% accuracy rate in predicting preterm preeclampsia was revealed in a study that was published in Nature Medicine. The test offers a non-invasive means of early detection by analyzing cell-free DNA released from the placenta. The goal of this development is to improve preventative treatment approaches for a disease that causes more than 70,000 maternal and 500,000 fetal deaths globally each year. Additionally, in May 2023, the FDA approved two blood tests developed by Thermo Fisher Scientific to help determine a pregnant woman’s risk of developing severe preeclampsia, a potentially fatal condition. These advancements highlight how important blood tests are to the changing field of maternal healthcare.
The urinalysis segment in the preeclampsia market is experiencing rapid growth, primarily due to its cost-effectiveness and simplicity. The segment has witnessed steady growth, due to the extensive use of urine protein tests to evaluate renal function and identify proteinuria, a defining feature of preeclampsia. In order to increase early detection rates, recent developments have concentrated on improving the sensitivity and specificity of urinalysis tests. Additionally, the combination of urinalysis and other diagnostic techniques has made thorough screening methods easier, which has improved outcomes for both mothers and fetuses.
Application Insights
In 2024, universal screening dominated the preeclampsia diagnostics market, primarily due to it becoming a standard procedure of prenatal care in most countries. The major role in its adoption has been met by nationwide screening programs and an increased emphasis on preventative healthcare. Authorities in health across the world are striving to equalize screening to enable that all pregnant women get evaluations in good time. These will further promote the use of universal screening and help to promote improved maternal health outcomes. International Federation of Gynecology and obstetrics (FIGO) recently pointed out the role of screening of preeclampsia during the first trimester. FIGO stipulates that pregnant women are supposed to undergo measurement of blood pressure and assessment of maternal risk factors during early stages of pregnancy. Biomarkers have been found to have potential in the early detection and treatment, but FIGO observes that further research is required to establish the efficacy in various populations and ethnic groups.
The targeted testing segment in the preeclampsia market is expected to grow at the fastest pace over the forecast period, reflecting the rising demand for personalized care in high-risk pregnancies. Targeted testing allows healthcare providers to tailor monitoring and interventions based on an individual’s specific risk profile, making it a highly effective approach. Advances in genetic testing and the identification of key risk factors have further enabled clinicians to implement more precise and timely monitoring protocols. Supporting this trend, a study published by the American Heart Association on May 6, 2024, showed that a personalized screening algorithm during the first trimester combining maternal history, ultrasound data, and blood biomarkers was more effective in predicting preeclampsia than traditional risk-based guidelines. The study, which included over 7,000 women, demonstrated that this method could facilitate earlier interventions and potentially reduce the occurrence of serious pregnancy complications. Additionally, integrating targeted testing with electronic health records and clinical decision-support systems has improved the efficiency and accuracy of preeclampsia management, helping providers deliver better, more timely care.
End Use Insights
The diagnostics center dominated the market in 2024, accounting for a 35.25% share and is also is anticipated to grow at the fastest rate over the forecast period. Diagnostic centers have become central to the preeclampsia diagnostics market, capturing a significant share due to their specialized services and advanced testing capabilities. These facilities offer a range of diagnostic tests, including blood and urine analyses, catering to the growing demand for early detection and monitoring of preeclampsia. In May 2024, Labcorp launched a first-trimester screening test for preeclampsia risk assessment, making it the only laboratory offering tests that cover all three trimesters of pregnancy. The growth of diagnostic centers, especially in urban areas, has made maternal healthcare more accessible to expectant mothers. Recent developments also show an increase in state-of-the-art diagnostic centers equipped with advanced technologies, further strengthening their role in early detection and effective management of preeclampsia.
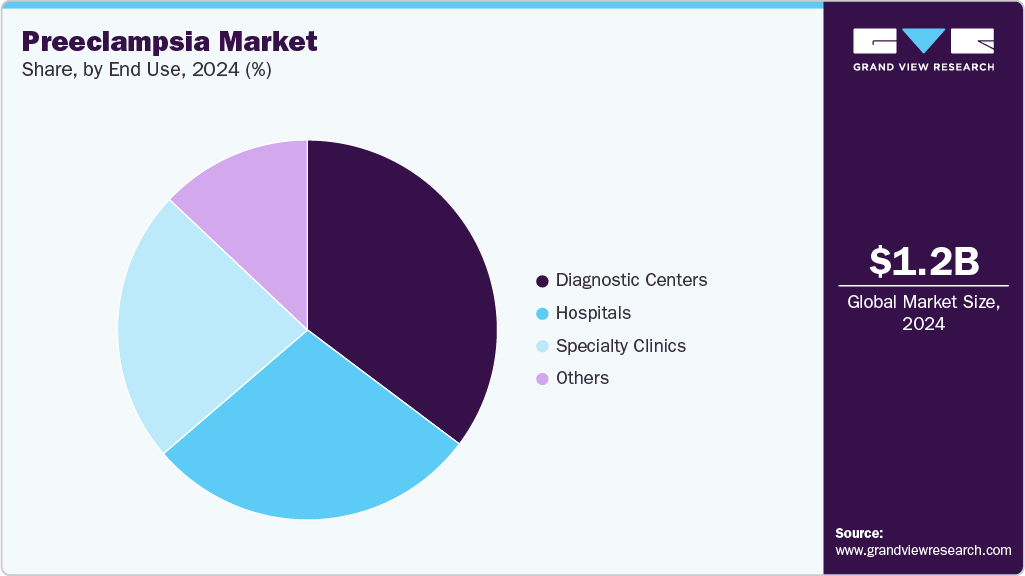
Hospitals segment is expected to grow at the considerable rate over the forecast period. Hospitals continue to play a crucial role in the management of preeclampsia, providing comprehensive care that encompasses diagnosis, monitoring, and treatment. The integration of multidisciplinary teams, including obstetricians, cardiologists, and neonatologists, ensures holistic management of preeclampsia cases. Recent trends highlight the adoption of advanced monitoring systems within hospital settings, allowing for real-time assessment of maternal and fetal well-being. These advancements contribute to timely interventions and improved outcomes for both mothers and infants.
Regional Insights
North America preeclampsia market dominated the global market and accounted for the largest revenue share of 40.66% in 2024, largely because of the growing interest in maternal health complications and the growing popularity of high-level diagnostic tools. Having well-developed healthcare systems, government programs that assist maternal treatment, and ever-developing new technologies of blood-based biomarker analysis are all contributing to this growth. The early diagnosis has also been improved by the introduction of first-trimester screening tests by companies in the region like Labcorp in 2024, which has additional stimulated the need to get a preeclampsia diagnosis.
U.S. Preeclampsia Market Trends
The U.S. preeclampsia market is growing incidence of preeclampsia. Preeclampsia complicates about 5% to 8% of U.S. births, a figure that has been steadily increasing. Black women are at a significantly higher risk, with a rate 60% higher than White women and a greater likelihood of poorer outcomes. Key risk factors include first pregnancy, a history of preeclampsia, chronic hypertension, diabetes, obesity, and carrying multiples. Furthermore, growing market approvals for test kits ia also driving the market. For example, in February 2025 Roche announced that its Elecsys sFlt-1/PlGF ratio for preeclampsia received FDA 510(k) clearance as a prognostic test to stratify hospitalized pregnant women with hypertensive disorders into low- and high-risk categories for developing severe preeclampsia within two weeks; Roche emphasized the test’s role as an objective adjunct to clinical assessment, and the clearance marked an important regulatory milestone for biomarker-based preeclampsia risk assessment.
Europe Preeclampsia Market Trends
Europe is experiencing strong growth in the preeclampsia market. Growth is supported by government-led initiatives to improve maternal health, well-established prenatal screening programs, and the adoption of both universal and targeted screening approaches. Recent guidelines from FIGO (2024) recommending universal screening have encouraged healthcare providers across the continent to standardize early detection practices, supporting market expansion. Whereas, In October 2025, NeoPredics announced the market launch of PreFree across CE-marked markets beginning in Q4 2025; PreFree is described as a CE-marked multimodal prediction model that combines biomarker inputs (including sFlt-1/PlGF) with clinical data to deliver risk stratification and remote monitoring capabilities for preeclampsia, positioning the company to expand clinical decision-support tools in maternal care across Europe.
The UK preeclampsia market is growing rapidly, supported by increasing hospital-based screening programs, integration of personalized risk assessment tools, and rising awareness among expectant mothers. Enhanced by the growing number of screening programs in hospitals, the inclusion of the customized risk assessments, and the growing awareness of the future moms. Market adoption has also been enhanced through public health campaigns on early detection. In July 2022 PerkinElmer announced that two of its placental growth factor (PlGF)-based diagnostics tests were found in the updated National Institute of Health and Care Excellence (NICE) guidelines on diagnosing suspected preterm pre-eclampsia in the UK. These tests-the DELFIA Xpress PlGF 1-2-3 kit and the DELFIA Xpress sFlt-1 kit-are now recommended for use alongside standard clinical assessments in women from 20 weeks of pregnancy onwards. PerkinElmer is the only diagnostics company to offer two different PlGF-based tests recommended in the updated guidance, providing healthcare providers across the UK with more options to help diagnose and initiate treatment for expectant mothers suspected of this condition.
Asia Pacific Preeclampsia Market Trends
The market of Asia-Pacific preeclampsia is developing rapidly due to the rising maternal population, the rapid urbanization, and the improvement of healthcare facilities. Developing countries like India and China are also improving the diagnostic and prenatal care service, and early detection and tracking are becoming more available. Advanced blood-based biomarkers, as well as the use of urinalysis and the use of specific screening techniques, are increasingly common in the city hospitals and diagnostics facilities. Meanwhile, the governmental programs to reduce maternal and newborn mortality are facilitating the implementation of early screening programs and increasing the budget on maternal healthcare, which in turn encourages the growth of the market.
The Japan preeclampsia market is gradually embracing preeclampsia, with growth supported by government support for maternal health programs and the increasing availability of advanced diagnostics in hospitals and clinics.
The China preeclampsia market is rapidly increasing due to the increased awareness of maternal complications, increased access to healthcare, and the development of novel biomarker-based tests in large hospitals. Most recently, in July 2024, Shuwen Biotech had one rapid, non-invasive, urine-based, point-of-care test of preeclampsia detection approved by the China National Medical Products Administration (NMPA). It is a self-test that was created under the name CercaTest RED, to be used by professionals in medical facilities and also by a person as a self-test to bring the results in five minutes. It uses a new mechanism of identifying misfolded proteins in the urine, a symptom that is a precursor of preeclampsia. In January 2025, Shuwen Biotech started the PreShield Study a multicenter clinical trial to determine the effectiveness of the CercaTest RED in self-testing conditions. The proposed study is to recruit 1,500 pregnant women diagnosed as being at risk of preeclampsia and these women will conduct their own serial self-testing during the course of their pregnancy. The aim is to evaluate the possibility of the test in early diagnosing and monitoring preeclampsia potential.
These advancements make Shuwen Biotech a leader in the field of preeclampsia diagnostics in China and provide new opportunities to improve early detection and treatment of the severe pregnancy complication.
Latin America Preeclampsia Market Trends
The Latin American preeclampsia market is witnessing moderate to high growth, fueled by increasing healthcare spending, expanding hospital networks, and rising awareness of maternal health complications. Countries such as Brazil and Argentina are investing in prenatal care programs and diagnostic testing infrastructure. Public and private healthcare providers are increasingly adopting advanced screening methods, including blood-based biomarker tests and urinalysis, to improve early detection rates. These measures aim to reduce maternal and fetal morbidity and mortality, which remains a key focus for governments and healthcare organizations in the region.
Middle East and Africa Preeclampsia Market Trends
The Middle East and Africa region shows strong potential for preeclampsia growth. The MEA preeclampsia market shows strong potential for growth, primarily driven by increasing awareness of maternal health, the expansion of hospital networks, and rising adoption of diagnostic testing. Governments in countries such as Saudi Arabia, the UAE, and South Africa are actively investing in maternal healthcare infrastructure and prenatal care programs. Private hospitals and diagnostic centers are also enhancing their capabilities by integrating advanced maternal-fetal health technologies.
The Saudi Arabian preeclampsia market is expanding rapidly, supported by national healthcare initiatives, the integration of cutting-edge diagnostic solutions in hospitals, and increasing patient awareness about prenatal care and early screening.
Key Preeclampsia Company Insights
The preeclampsia market is driven by a mix of established diagnostics companies and specialized innovators focused on expanding access and ease of assays. Major players shaping this landscape include: Revvity, Thermo Fisher Scientific Inc., Metabolomic Diagnostics Ltd. (Trinity Biotech), Sera Prognostics (U.S.), Siemens Healthineers AG and others
Key Preeclampsia Companies:
The following are the leading companies in the preeclampsia market. These companies collectively hold the largest market share and dictate industry trends.
F. Hoffmann-La Roche Ltd
Revvity
Thermo Fisher Scientific Inc.
Diabetomics, Inc.
Metabolomic Diagnostics Ltd. (Trinity Biotech)
Sera Prognostics
Siemens Healthineers AG
Bayer AG
Quidel Corp
Eurofins NTD Genetics
mProbe’s
Labcorp
Recent Developments
In October 2025, Mirvie announced the results of its novel, non-invasive blood test designed to predict a woman’s risk of developing preeclampsia during pregnancy. The test analyzes molecular signals in maternal blood to identify early risk factors before clinical symptoms appear, aiming to enable timely preventive care and improved maternal outcomes. The findings underscore the potential of precision diagnostics in transforming prenatal care by shifting from reactive to predictive approaches in preeclampsia management.
In August 2025, Trinity Biotech announced the commercial launch of an FDA-cleared preeclampsia testing service (the PreClara Ratio sFlt-1/PlGF test) in collaboration with Thermo Fisher, offering the biomarker ratio as a reference-lab service to support timely risk stratification and clinical decision making for hypertensive disorders of pregnancy in the U.S.; Trinity positioned the service to expand access to an FDA-cleared diagnostic while leveraging its Immco reference laboratory and commercial infrastructure.
In February 2025 Roche announced that its Elecsys sFlt-1/PlGF ratio for preeclampsia received FDA 510(k) clearance as a prognostic test to stratify hospitalized pregnant women with hypertensive disorders into low- and high-risk categories for developing severe preeclampsia within two weeks; Roche emphasized the test’s role as an objective adjunct to clinical assessment, and the clearance marked an important regulatory milestone for biomarker-based preeclampsia risk assessment.
In September 2024, Trinity Biotech announced the acquisition of Metabolomics Diagnostics (developer of the PrePsia early-pregnancy predictive test) for a modest enterprise value, acquiring mass-spectrometry and machine-learning capabilities to commercialize PrePsia (first-trimester risk prediction for preeclampsia) via Trinity’s Immco reference lab in the U.S. and to pursue international rollout, with the company stating first revenues from the test were expected in 2025.
In January 2024, Labcorp introduced the BRAHMS sFlt-1/PlGF KRYPTOR Test System, the first FDA-cleared blood test designed to assess the risk of progression to severe preeclampsia in pregnant women. This test measures the ratio of two angiogenic biomarkers-soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF)-to help clinicians identify patients at high risk of developing severe preeclampsia within two weeks. This advancement addresses the limitations of traditional diagnostic methods, such as blood pressure and proteinuria evaluations, which have been shown to be inadequate predictors of severe adverse maternal and perinatal outcomes
Preeclampsia Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 1.36 Billion
Revenue forecast in 2033
USD 2.73 Billion
Growth Rate
CAGR of 9.12% from 2025 to 2033
Base year for estimation
2024
Historical data
2021 – 2023
Forecast period
2025 – 2033
Quantitative units
Revenue in USD Billion/Million and CAGR from 2025 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Test Type, application, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Norway; Sweden; Denmark; Japan; China; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
F. Hoffmann-La Roche Ltd; Revvity; Thermo Fisher Scientific Inc.; Diabetomics, Inc.; Metabolomic Diagnostics Ltd. (Trinity Biotech); Sera Prognostics (U.S.); Siemens Healthineers AG; Bayer AG; Quidel Corp; Eurofins NTD Genetics; mProbe’s; Labcorp
Customization scope
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Preeclampsia Market Report Segmentation
This report forecasts revenue growth and provides an analysis of the latest trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the global preeclampsia market based on test type, application, end use, and region:
Test Type Outlook (USD Million; 2021 – 2033)
Application Outlook (USD Million; 2021 – 2033)
End Use Outlook (USD Million; 2021 – 2033)
Hospitals
Specialty Clinics
Diagnostic Centers
Others
Regional Outlook (USD Million, 2021 – 2033)
North America
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
