SCOTTSDALE, Ariz. – Nearly every study shows that teen depression is only increasing. Now, the Food and Drug Administration (FDA) is giving families another tool to fight severe depression.
The new tool is not a medication, but a device that uses magnetic pulses to stimulate the brain.
By the numbers:
According to the National Institute of Mental Health, 20.1% of teenagers in the U.S. had at least one major depressive episode. At the same time, they estimate only 40.6% of them received any treatment.
What we know:
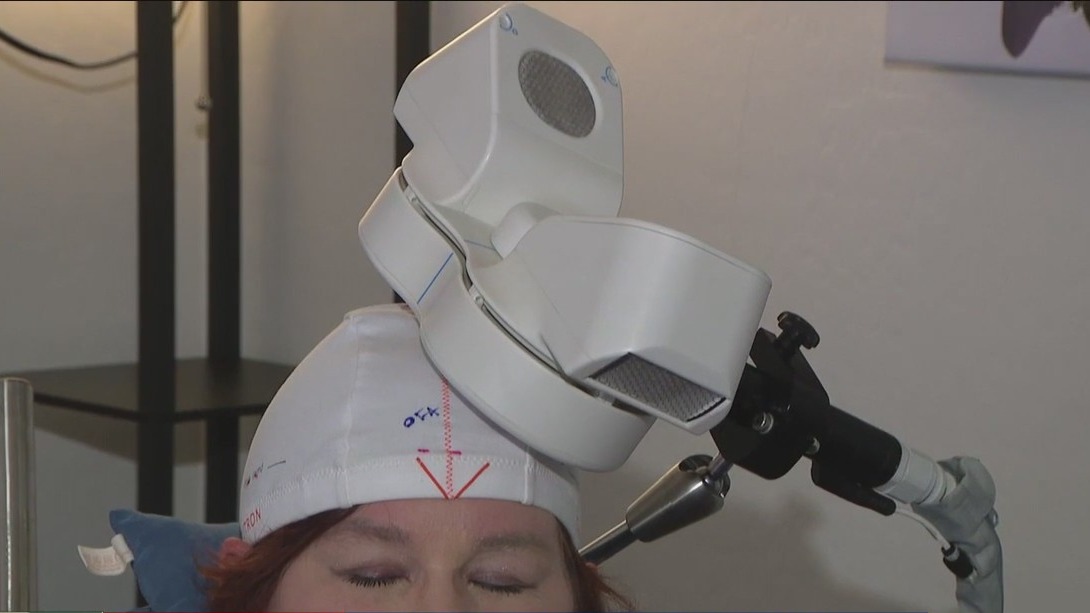
The treatment process starts with a cap created specially for each patient.
“The treatment is quite targeted, so you can see this dot F3. So this is measured off an XY-coordinate that shows exactly where to place the paddles,” Juliane Popelka of American TMS Clinics said.
Popelka got her patient ready at American TMS Clinics in Scottsdale for her Transcranial Magnetic Stimulation (TMS).
“We match up that bullseye with the target zone and then we get them started,” Juliane said.
The magnetic pulses fire up parts of the brain, generating a clicking noise.

Local perspective:
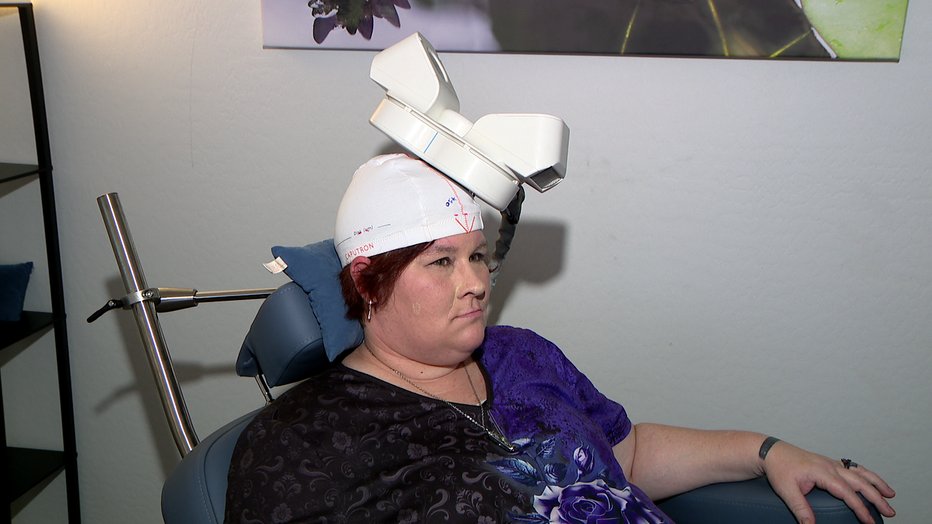
“For me, it feels very calming,” said Margaret Wilson.
Wilson has tried every medication for depression she can think of. Once she tried TMS, an FDA-approved therapy, she said everything changed.
“I just laughed one day. He said something funny and normally I’m like… but this day I started laughing and I’m like, ‘Wait a minute, I’m laughing. Oh my God.’ It made me cry,” Wilson said.
Adults have had access to this non-conventional therapy for years, but now, the FDA has approved TMS for kids as young as 15.
For many, that means insurance will cover it, which is important because the cost of a minimum of 36 treatments can add up.
What they’re saying:
“For teens who are in desperate need of care and have tried everything and their parents have tried everything, they finally have some hope and it’s not going to cost them a second mortgage,” said Popelka.
“When something gets approved by the FDA, there is a degree of relief that people feel that this treatment has been studied, has been researched,” added Dr. Houshang Aminian.
Dig deeper:
The most common side effect is a headache. Often, they say TMS is chosen after therapy and medications haven’t worked.
“It’s changed my life,” Wilson said.
Now that Wilson knows it’s FDA-approved for teens, she wishes it happened earlier. Her symptoms started when she was just 15.
“It would have changed, it would have helped give me my life back,” she said.
The Source: This information was provided by a technician and doctor at American TMS Clinics.

