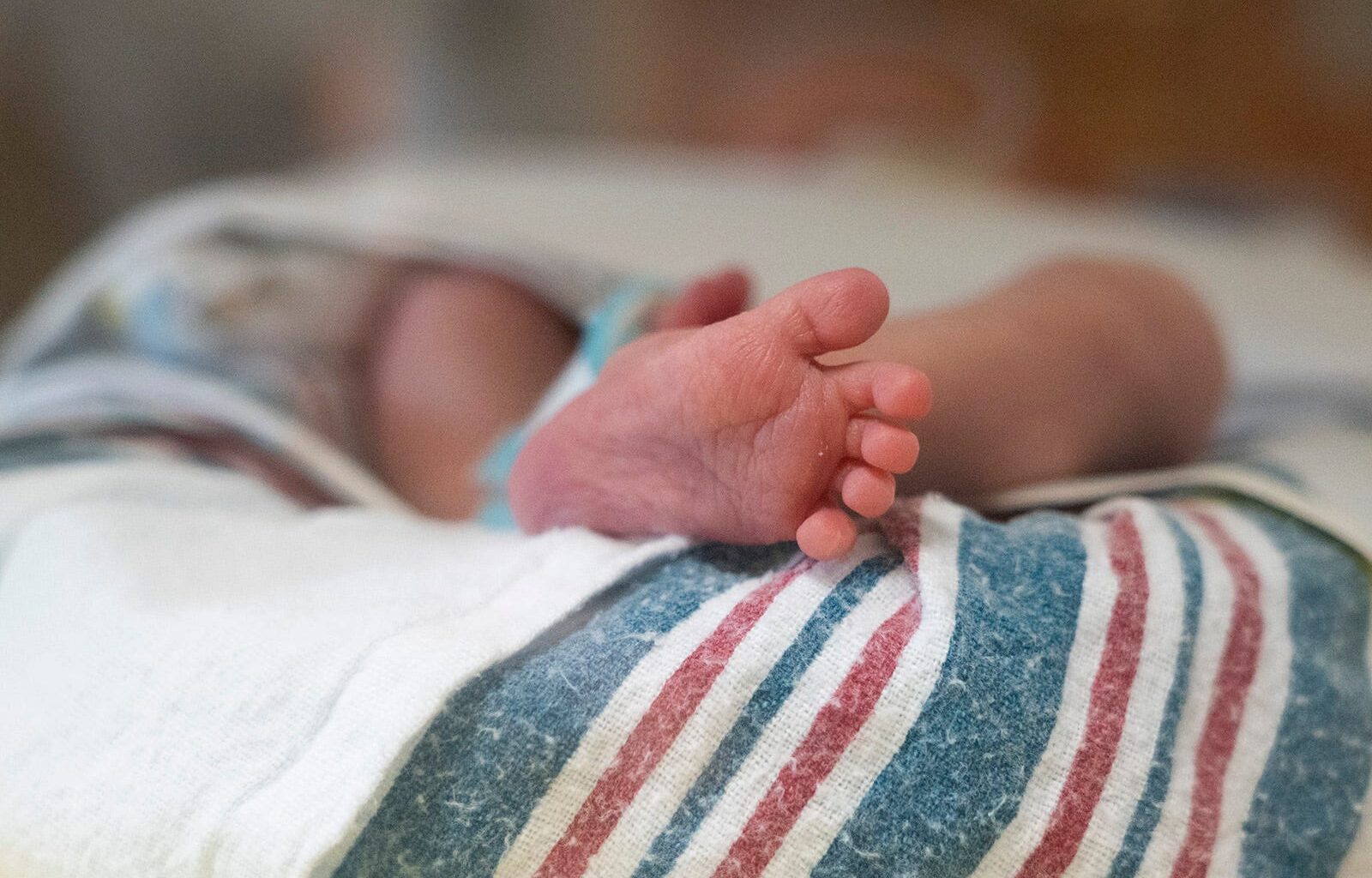
Newborn screenings in Texas now test for 60 genetic conditions that have treatments that can be given. Four conditions were added in August.
Mikala Compton/American-Statesman
Dr. James Gibson and his genetics clinic at Dell Children’s Medical Center are expecting a first any day now: The first baby with a lysosomal storage disorder to come to the clinic for treatment before they show signs of disease.
Starting on Aug. 18, newborns in Texas are now screened for four new disorders:
Article continues below this ad
Glycogen storage disease Type II (Pompe)
Mucopolysaccharidosis Type I (MPS I)
Mucopolysaccharidosis Type II (MPS II)
Infantile Krabbe disease (Krabbe)
The screening, which now includes 60 disorders, happens as a heel prick blood test when a newborn is still in the hospital, usually at a day or 2 days old, and then again at their two-week pediatrician visit.
If you have never heard of these disorders, they are rare. Gibson expects there might be only one to four babies born in Texas with each of these disorders each year, but the effects of these conditions are devastating.
What is missing in these disorders?
All of these lysosomal storage diseases cause the body to lack a certain enzyme. A toxic buildup in the tissues and organs then occurs. Not having those enzymes can quickly impact the central nervous system, including the brain. For years, children with these disorders would have delays, regress or never gain skills including walking, talking, feeding themselves or using the bathroom. The impact to the brain eventually leads to death.
Article continues below this ad
For some of the diseases, like the infantile form of Pompe, without therapy, the child might live a year or slightly longer. For Krabbe, it would be rare to live beyond age 3 without therapy, and treatment would also need to be done within weeks of being born to avoid permanent damage. As Gibson explains, once these disorders affect the central nervous system, even new treatments cannot reverse the damage. Treatments can just prevent future damage.
Newborn screening changes the nature of these diseases. “If we can find these diseases early, the outcome is better,” Gibson said. Therapies can be started within weeks of a baby being born, he said, or that child could be monitored if it’s suspected they have a form of the disease that has a later onset.
Previously, to get diagnosed with the disorder, a parent would wait until they sensed something is wrong and go to their pediatrician. The pediatrician might wait to see if the symptoms continue and then give the parent a referral to a neurologist. That neurologist would then reach out to a geneticist for testing. Months would go by from the time a parent was worried to the time a diagnosis was confirmed and treatment would begin. Damage to the brain already might have occurred.
Senior scientist Neda Ghosifam looks at the newly separated cells through a microscope at the lab on Austin Community College Highland campus in 2023. She is part of a team of people looking for a treatment for rare genetic disorders. Finding treatments allow disorders to be part of the newborn screening.
Kara Hawley / American-Statesman
Why not screen before?
Adding these conditions to the newborn screening would not have been possible years before when no treatments were available that the public found acceptable, Gibson said.
Article continues below this ad
Now, there are actual treatments that either give the children the missing enzyme through an infusion at regular intervals such as every two weeks or the child receives a stem cell transplant, in the case of Krabbe.
“Therapy is not benign,” Gibson said.
Infusions can mean a treatment every two weeks for the rest of their life. A stem cell transplant requires a family to move to another city for a few months because Dell Children’s is still building its stem cell transplant center. The gene code is altered permanently in the hopes that the body will soon have the enzyme. In some cases, a stem cell transplant followed by enzyme replacement might be necessary.
“Therapies are not perfect, but they extend life expectancy and they extend the quality of life,” Gibson said.
Article continues below this ad
Tim Revell and his wife, Laura, seen here in 2015, have been trying to fund research and treatments for Duchenne muscular dystrophy, which both their sons Timothy and Andrew have. This is one of the newest diseases being considered by the federal government to put on a list of disorders for states to consider for newborn screenings. Duchenne’s now has a treatment that wasn’t available when the Revell boys were born.
Andy Sharp
Waiting for federal guidance
Newborn screening began in 1963 with one disorder, phenylketonuria, which was linked to many cases of intellectual disabilities. Now every year, Texas identifies about 1,000 newborns with a treatable genetic disease. It has been four years since the last disease (spinal muscular atrophy) was added to the screening. Texas law now requires the state to follow the federal Recommended Uniform Screening Panel recommendations when funding is available.
To get on the RUSP list, the federal government’s Advisory Committee on Heritable Disorders in Newborns and Children considers evidence that a disease is something that can be screened for in a newborn. That committee has long held that it will only screen for conditions that have a treatment that will make a measurable difference when given early.
Article continues below this ad
Once the committee approves a disorder to be added to the RUSP list, it then needs to be approved by the secretary of the U.S. Department of Health and Human Services.
Then each state decides which and when to add a disorder to its newborn screening roster. The state has to develop the testing protocol, train the laboratory technicians and calibrate testing machines. Each condition can require different lab equipment, reagents and supplies.
The state also has to develop a communication plan about the new test and assemble resources for families and pediatricians. The cost of the screenings, which are paid by private insurance, Medicaid or a charity program, increase with each screening. This year, the test costs $68.63, but is expected to increase in 2026.
Article continues below this ad
“We can’t blink twice and snap our fingers and have everything involved in place,” said Debra Freedenberg, who was the medical director of the newborn screening unit and genetics at the Texas Department of State Health Services when spinal muscular atrophy was added four years ago.
More screenings could be coming soon: The federal advisory committee is seeking public comments through Sept. 15 on adding Duchenne muscular dystrophy and Metachromatic leukodystrophy.