BORS is a rare autosomal dominant genetic disorder with significant heterogeneity in clinical practice, and the penetrance rate is highly variable even within the same family. Research has shown that BORS exhibits hearing loss (noticed in 98.5% of cases), gill abnormalities (49–73%), preauricular depression (53–83%), and renal abnormalities (38–70%) [4, 5]. In addition to the typical clinical manifestations of ears, gills, and kidneys mentioned above, many studies have also reported other accompanying symptoms of BORS, such as developmental delay, intellectual disability, hypospadias, and bone defects. [6,7,8]. Hearing loss is the most common symptom of BORS, with 64–100% of patients experiencing some degree of hearing loss [3, 6, 9,10,11]. Mixed hearing loss is the most common type of hearing loss in patients with BORS (33.7–50%), followed by sensorineural (10.98–29.8%) and conductive (7.84–30%) hearing loss [6, 11,12,13]. Imaging studies have shown that structural abnormalities in the inner/middle ear, such as underdeveloped cochlea and enlarged vestibular aqueduct, are closely related to the progression of hearing impairment. Imaging studies can detect abnormalities in the inner ear (18–92%) and/or middle ear (15–100%) of BORS [9, 14]. Cochlear hypoplasia is the most common inner ear abnormality (33–100%), followed by vestibular aqueduct enlargement (24–50%) and inner ear canal abnormalities (17–86%) [13, 15, 16]. Kemperman analyzed long-term series audiometry data of patients with BORS, and the results showed that patients with BORS with large endolymphatic vessels and/or cysts on MRI seemed more likely to develop more severe hearing impairment. Furthermore, in clinical practice, about 70% of patients with hearing loss do not progress, and 30% of patients with progressive hearing loss often suffer from vestibular aqueduct enlargement [17].
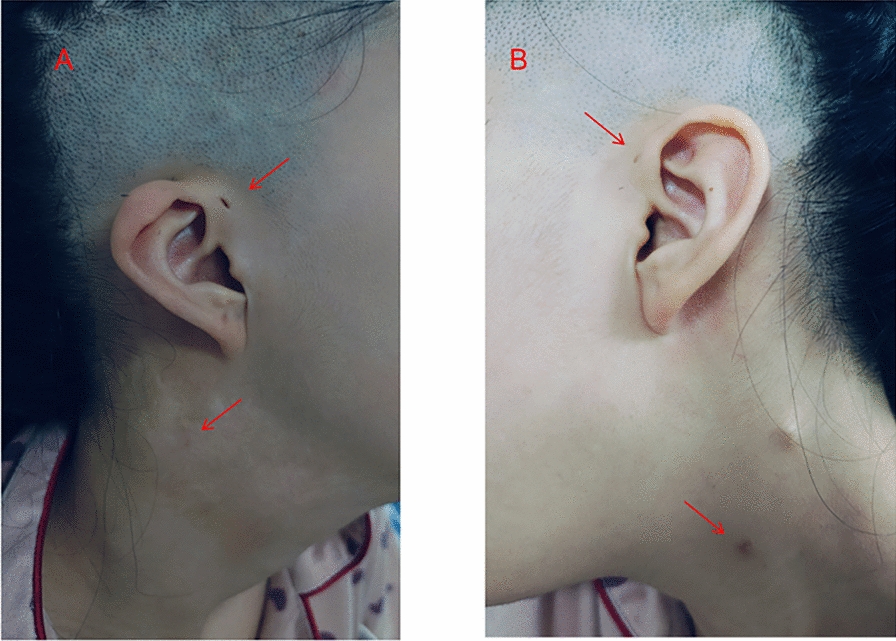
The patient presented with bilateral branchial fistula, preauricular fistula, auricular malformation, large vestibular aqueduct, and left renal dysplasia, which meet the diagnostic criteria of BORS. For this patient, we removed both fistulas separately, ensuring the integrity of the anatomical structure while reducing the risk of fistula recurrence. There is no cure for BORS in patients with sensorineural hearing loss, and wearing hearing aids can be used as an adjuvant treatment. For patients with BORS who cannot obtain good hearing assistance from hearing aids, cochlear implantation is expected to serve as an alternative approach, enabling patients with advanced hearing impairment to achieve a good hearing prognosis. In this case report, the patient suffered from bilateral sensorineural hearing loss, but the patient consciously did not have significant hearing loss, which may be related to the fact that the patient has adapted to hearing loss. Patients may need to use hearing aids or cochlear implants to further improve hearing. In addition, the patient also has an enlarged vestibular aqueduct, which predisposes them to progressive hearing loss. Therefore, hearing should be checked at least every 6 months, and ototoxic medications should be avoided to prevent further hearing deterioration. BORS exhibits high heterogeneity in renal malformations, with clinical manifestations ranging from mild structural abnormalities to end-stage renal disease. The latest research shows that about 10–20% of patients with BORS have renal abnormalities, including renal dysplasia (such as volume reduction or structural disorder), unilateral/bilateral kidney absence, polycystic kidney disease, ureteral dilation, or hydronephrosis [18]. Among them, 6% of cases present with severe renal malformations (such as bilateral renal hypoplasia), which may progress to chronic renal failure within 20 years after birth and require dialysis or kidney transplantation [19]. Severe kidney deformities can also lead to Potter’s facial features, including wide eye distance, low ear position, mandibular retraction, and flat nose [20]. In this case, the patient had unilateral renal dysplasia and required regular monitoring and management of renal abnormalities, including at least biannual ultrasound examinations and estimated glomerular filtration rate (eGFR) monitoring, to prevent the progression of end-stage renal disease. In recent years, significant progress has been made in the genetic research of BORS. Research has found that mutations in the EYA1, SIX1, and SIX5 genes are closely related to BORS, with the EYA1 gene being the most common pathogenic gene [3]. In the European and American populations, about 40% of patients carry this gene mutation [3, 21]. The protein encoded by the EYA1 gene plays an important role in embryonic development, especially in the development of gill arches, ears, and kidneys [9]. Research has shown that the EYA1 gene regulates organogenesis. Mutations in human EYA1 can lead to BORS, while targeted inactivation of mouse EYA1 can impair early development of multiple organs.. In addition, EYA1 is also involved in controlling key early induction events involved in thymus, parathyroid, and thyroid morphogenesis [22]. In addition to BORS, the EYA1 gene is also closely related to the occurrence and development of stem cell carcinoma, melanoma, breast cancer, and other tumors [23,24,25].
About 4% of patients with BORS carry the SIX1 gene mutation [26]. The SIX1 gene is regulated by the EYA1 gene, and the SIX1–EYA1 protein complex affects organ development during embryonic development by regulating cell proliferation and differentiation [27]. Recent studies have found that the SIX1 protein is involved in the regulation of expression patterns during early sensory development, and mutations can affect embryonic craniofacial gene expression and auditory sac development [28, 29]. In clinical practice, patients with gill kidney lineage diseases carrying SIX1 pathogenic mutations are more prone to severe sensorineural hearing loss [30]. About 5% of patients with BORS carry a pathogenic mutation in the SIX5 gene. Hoskins et al. first discovered mutations in SIX5 in patients in 2007 and demonstrated that mutations affect the transcriptional activation of the SIX5 protein and SIX5–EYA1 protein complex, indicating that SIX5 is a BORS-related gene [31]. At present, it is believed that the pathogenic mechanism of SIX5 mutation is similar to that of the SIX1 mutation. However, no SIX5 mutation was found in the subsequent large-scale screening, and some cases were re attributed to EYA1 mutation, therefore its pathogenicity is questionable.
In addition to the above three genes, nearly half of the patients still need to further explore the genetic basis of their pathogenic factors. Currently, new genes related to BORS are constantly being discovered. In 2014, Morisada reported a sporadic case of BORS in Japanese patients and found through array comparative genomic hybridization that a 6 Mb microdeletion on chromosome 16 of the SALL1 gene was associated with the BORS phenotype [32]. SALL1 mutations are usually associated with Townes Brocks syndrome (manifested as anal atresia, ear deformities), partially overlapping with the clinical phenotype of BORS, but whether the pathogenic mechanism is directly related still needs to be verified [33]. In 2013, Brophy analyzed the whole genome copy number variations of 35 patients with BORS who did not have EYA1, SIX1, or SIX5 gene mutations and found that 17 of them had significant copy number variations (11 chromosomal microdeletions and 6 microduplications), thus identifying the chromosomal hotspots of BORS and discovering that the SHARPIN, FGF3, and HOXA gene clusters were potential pathogenic genes [21].
Genetic testing, such as multiplex ligation-dependent probe amplification (MLPA) and next-generation sequencing (NGS), has significant value in the diagnosis of BORS. MLPA can detect copy number variations in the EYA1 gene, while NGS can comprehensively screen for mutations in the EYA1, SIX1, and SIX5 genes. Despite the high cost of genetic testing, its potential application prospects in the diagnosis and genetic counseling of BORS are broad. Future research should further explore the application value of genetic testing in BORS diagnosis, especially in situations where resources are limited. Unfortunately, since the patient’s family refused to proceed with further genetic testing, the type of gene mutation in this patient could not be verified. The lack of genetic testing not only confines the diagnostic basis of this case to clinical manifestations and imaging assessments but also significantly restricts the precise evaluation of genetic risks for the patient and their family. Confirmation of pathogenic variants in EYA1 or related genes would not only strengthen the diagnostic basis for BORS but also enable targeted genetic screening for other potential carriers in the family (such as parents and siblings). This would facilitate the early identification of recessive carriers and allow for risk communication, prenatal diagnosis, or genetic screening during their family planning, thereby significantly altering the family’s reproductive decision-making and risk management strategies. Meanwhile, genetic testing can also guide the multidisciplinary team in developing more personalized follow-up and intervention plans. Since genetic testing was not performed in this case, it has, to some extent, limited the long-term management of the case and the provision of precise genetic counseling to the family members.