A year ago, a team of scientists uncovered an unusual discovery. Just 4,000 years ago, when the first human civilizations had established themselves in Mesopotamia and Egypt, mammoths were still alive on Wrangel, a remote Arctic island. This Tuesday, the same team published an exhaustive analysis of hundreds of mammoth remains spanning a million years, with a new surprise: they were able to isolate DNA from the bacteria that lived inside these animals from their teeth and other tissues. This discovery opens up a unique opportunity to understand the co-evolution of mammoths and their microbiome, and perhaps shed light on the causes of their extinction.
Some of the scientists behind the study work for the U.S. company Colossal, which aims to de-extinct the mammoth by 2027.However, many experts believe this project will never truly bring these animals back, but rather create strange, reddish-haired elephants. This new study could also make it possible to recover the bacteria that coexisted with the pachyderms — and may have caused them deadly infections.
The researchers analyzed 483 mammoth remains, mostly molars but also tusks and bones. The oldest samples are 1.1 million years old and belonged to a steppe mammoth — a species that later gave rise to the woolly mammoths of Europe and the Columbian mammoths of the Americas — that lived in Adycha, in the Arctic region of present-day Russia.
The researchers recovered genetic material from six groups of bacteria, some capable of causing severe infections. The bacterial groups identified in mammoths are related to modern microbes that either live peacefully with their hosts or cause health problems, such as streptococci linked to dental cavities. Among them are Pasteurella, related to present-day microbes capable of killing African elephants — the mammoths’ closest living relatives, alongside Asian elephants — through septicemia. The study was published Tuesday in the journal Cell.
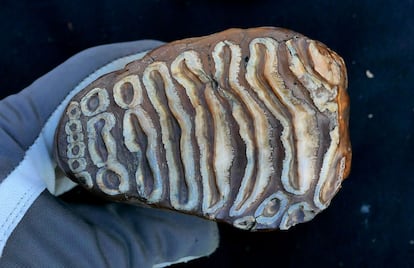 Una de las muelas de mamut analizadas en el estudio.Peter Mortensen
Una de las muelas de mamut analizadas en el estudio.Peter Mortensen
The oldest sample preserves remains of the genome of Erysipelothrix bacteria. Today, these microbes are found in the mouths of pigs or dogs. They can enter the bloodstream and cause serious infections such as endocarditis, which inflames the tissue lining the heart. In the mammoth remains, these bacteria appear in the bones. This could mean that there were infections that caused health problems in these animals, although it could also represent a benign colonization. For now, it is impossible to know.
Bioinformatician David Díez del Molino, co-author of the study, explains the difficulties posed by some of the samples. Some of the molars studied — including the oldest one — were discovered in the permafrost in the 1970s. Some contain so little mammoth DNA that nothing had been published from 440 of them until now.
“We’ve been sequencing mammoths for more than 10 years,” explains this scientist, which in part has helped reveal the DNA of the bacteria associated with many of those remains.
The results push the study of microbial DNA back one million years, says biologist Benjamin Guinet of the Centre for Palaeogenetics at Stockholm University and the Swedish Museum of Natural History. These are by far the oldest known. The finding “opening up new possibilities to explore how host-associated microbes evolved in parallel with their hosts” over thousands of years, the researcher stressed in a press release from his university.
The lead author of the study is geneticist Love Dalén, one of the world’s foremost experts on mammoth genetics. The researcher has worked on recovering the mammoth DNA, which was so well preserved by Siberian cold, it retained its three-dimensional structure. This is a key finding for understanding which genes were active in these creatures, especially those related to their physical traits and their extraordinary adaptation to icy environments. With such data, it would theoretically be possible to reproduce them in the genome of modern elephants in order to bring the mammoth back.
“This work opens a new chapter in understanding the biology of extinct species,” Dalén explains in the statement. “Not only can we study the genomes of mammoths themselves, but we can now begin to explore the microbial communities that lived inside them.”
Dalén is a scientific advisor for Colossal, the U.S. company that recently created woolly mice with striking reddish fur and other traits by inserting mammoth genes into their genetic code. Four months ago, the company announced it had de-extincted an animal for the first time: the dire wolf (Canis dirus), which disappeared more than 10,000 years ago.
The scientists started from a reconstruction of the extinct animal’s genome and then edited gray wolf cells to match that of the vanished canid. The announcement was controversial, since many experts believe this does not amount to truly de-extincting an animal, but rather creating a new variant of present-day wolves that resemble dire wolves through genetic editing.
The U.S. company was co-founded by charismatic biologist George Church of Harvard University, a pioneer of human genome sequencing and a champion of genetic editing’s potential to prevent diseases and even enhance the abilities of animals and humans. Colossal has raised hundreds of millions of dollars from backers such as Thomas Tull, producer of the film Jurassic World, and the famous heiress Paris Hilton. Another co-author of the new study is U.S. molecular biologist Beth Shapiro, the company’s chief science officer.
Argentine geneticist Nicolás Rascován, a researcher at the Pasteur Institute in Paris, was one of the study’s international reviewers. “This work is important because it shows how far we can go with ancient DNA; even to better understand the interactions between microbes and large extinct mammals, and to study how the microbiota may have influenced their adaptation or decline,” he says.
The specialist, who recently isolated plague bacteria from human remains to reconstruct their evolution in Europe and America, cautions that the data presented are not enough to know to what extent the mammoth bacteria were “commensals” that caused no health problems, or whether they were involved in the death of some animals. In any case, he adds: “The main value of the study is that it opens the door to exploring the microbiota of extinct species and asking ourselves what their microbial ecology was like.”
Could these bacteria be de-extincted using present-day microbes? “These types of ancient microbial genomes are highly fragmented,” with only a small percentage of their complete genome preserved, explains Rascován. This means that “they are nowhere near being de-extinct, nor is it feasible to reintroduce them, nor does it even make biological sense; since many related strains of these species exist today, and there is no evidence to suggest that those from the mammoth would be better adapted to this animal or confer any particular advantage or disadvantage,” he explains.
Rascován adds: “The study does raise interesting questions about what would happen if ancient bacteria with pathogenic potential were one day recovered, but we are still very far from that scenario.”
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition

