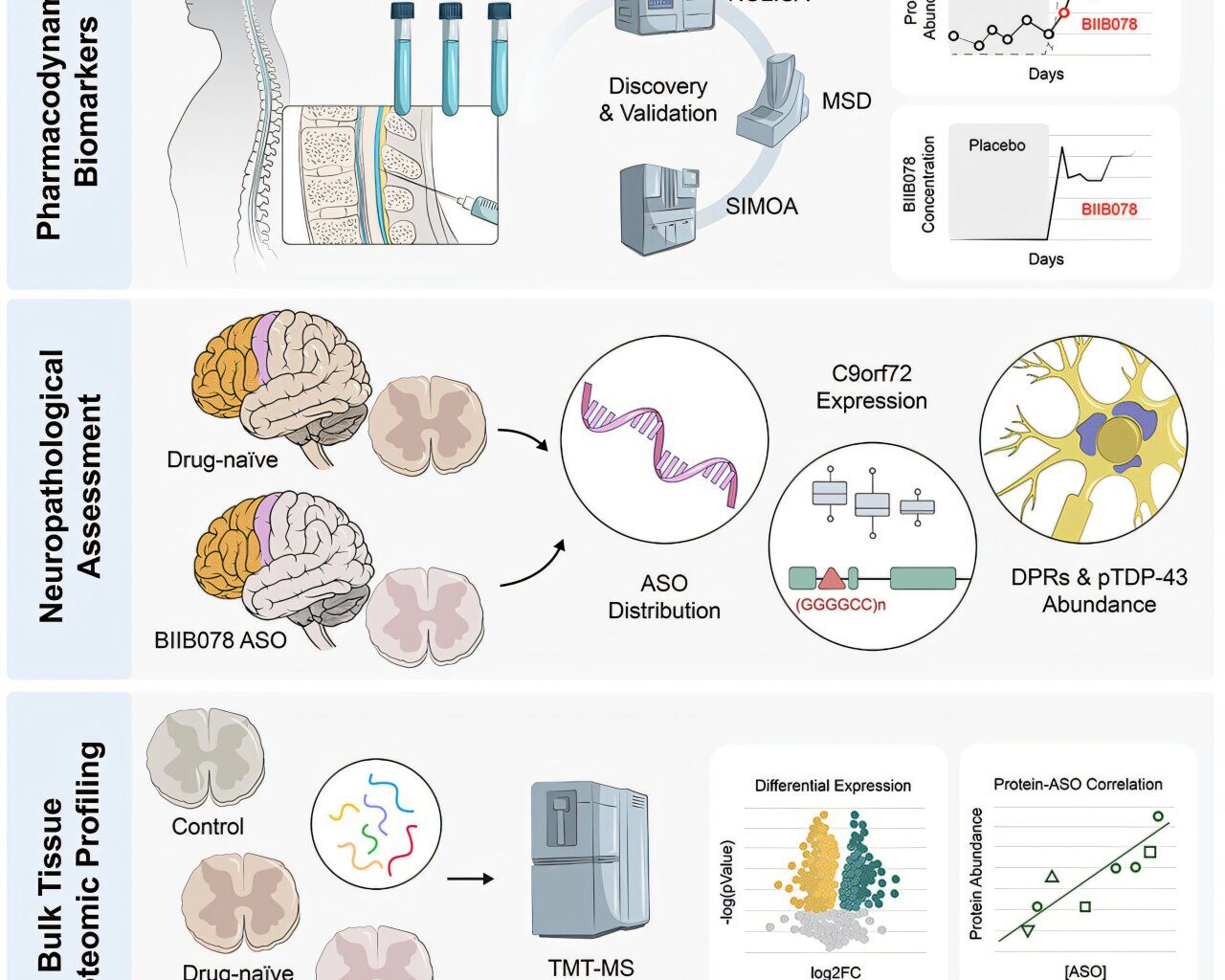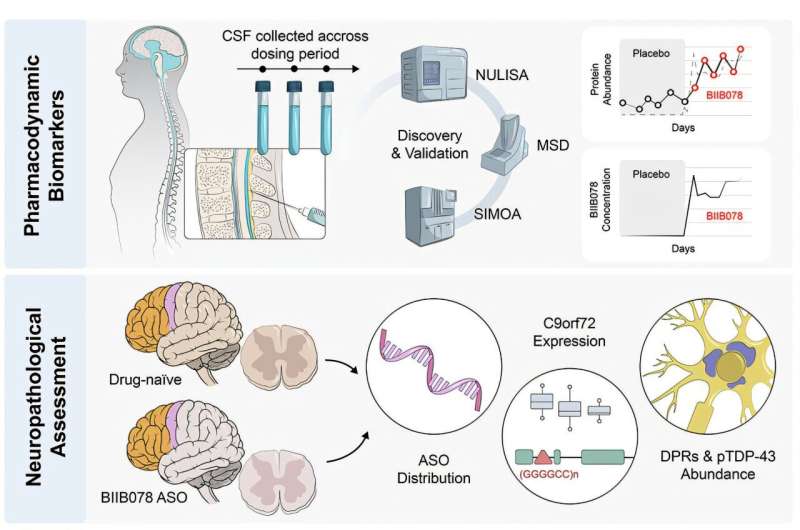
Graphical abstract. Credit: Cell (2025). DOI: 10.1016/j.cell.2025.07.045
A new Emory University study, led by the Emory ALS Center and the Center for Neurodegenerative Disease at Emory’s Goizueta Brain Health Institute, sheds light on why a once-promising experimental medication for amyotrophic lateral sclerosis (ALS) failed to help patients, despite successfully reaching its intended target in the central nervous system (CNS)—the brain and spinal cord.
The research is published in the journal Cell.
The therapy, called BIIB078, is an antisense oligonucleotide (ASO), a short strand of synthetic genetic material designed to block the production of toxic RNA and proteins in people with a genetic form of ALS linked to the C9orf72 gene. This genetic mutation is the most common inherited cause of the disease. A clinical trial of BIIB078 was stopped in 2021 after patients showed no clinical improvement.
“Disease-specific biomarkers in the cerebrospinal fluid (CSF) suggested that the ASO was hitting its intended target, but it was not clear if it penetrated CNS tissue and affected the disease itself,” says Zachary McEachin, Ph.D., assistant professor of Human Genetics and lead and co-senior author.
“Getting the drug to the CNS is only part of the challenge. We need better ways to know whether a treatment is truly changing the course of ALS. Our study addresses some of those questions and shows that CSF biomarkers do not always reflect changes in the CNS.”
In this new analysis of samples from trial participants, researchers examined CSF samples collected from participants during life, along with postmortem brain and spinal cord tissue. The study included eight individuals with C9orf72-linked ALS (c9ALS) who received BIIB078 and 31 ALS patients who did not receive the drug.
They found the drug spread widely throughout the central nervous system and reduced some of the toxic proteins linked to ALS. However, it did not improve or reverse the key disease processes that drive ALS, such as the buildup of abnormal brain proteins.
“This work is an important step forward to better understanding the biology of ASO therapies in people with neurological diseases,” says co-senior author Jonathan Glass, MD, director of the Emory ALS Center and professor in the Department of Neurology, Emory University School of Medicine.
“Several ASO therapies are either now in clinical use or in trial. We expect our new data will be influential in the development of new ASO-directed therapies.”
Investigators noted variability among patients in their biological responses to the ASO treatment, demonstrating the need to understand how individuals react to therapeutic interventions, an important issue for the goal of precision and personalized medicine. Investigators at the ALS Center are members of the Center for Neurodegenerative Diseases at the Goizueta Brain Health Institute.
“This work utilized integrated proteomic approaches to rigorously evaluate molecular changes in both tissue and CSF following ASO therapy, providing new biomarkers of treatment and insights to better design and monitor future clinical trials,” says Nicholas Seyfried, Ph.D., Professor of Biochemistry and co-senior author.
ALS, also known as Lou Gehrig’s disease, is a progressive condition that attacks nerve cells controlling muscle movement. Most people with ALS live only two to five years after diagnosis, and effective treatments remain limited. While the majority of ALS cases are sporadic, this study focused specifically on the less common genetic form, which accounts for approximately 10% of all cases.
The findings highlight the urgent need for new tools, known as biomarkers, that can show in real time whether experimental therapies are meaningfully impacting the disease.
More information:
Zachary T. McEachin et al, Molecular impact of antisense oligonucleotide therapy in C9orf72-associated ALS, Cell (2025). DOI: 10.1016/j.cell.2025.07.045
Journal information:
Cell
Provided by
Emory University
Citation:
Why a promising ALS drug failed—despite hitting its target (2025, September 9)
retrieved 9 September 2025
from https://medicalxpress.com/news/2025-09-als-drug.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.