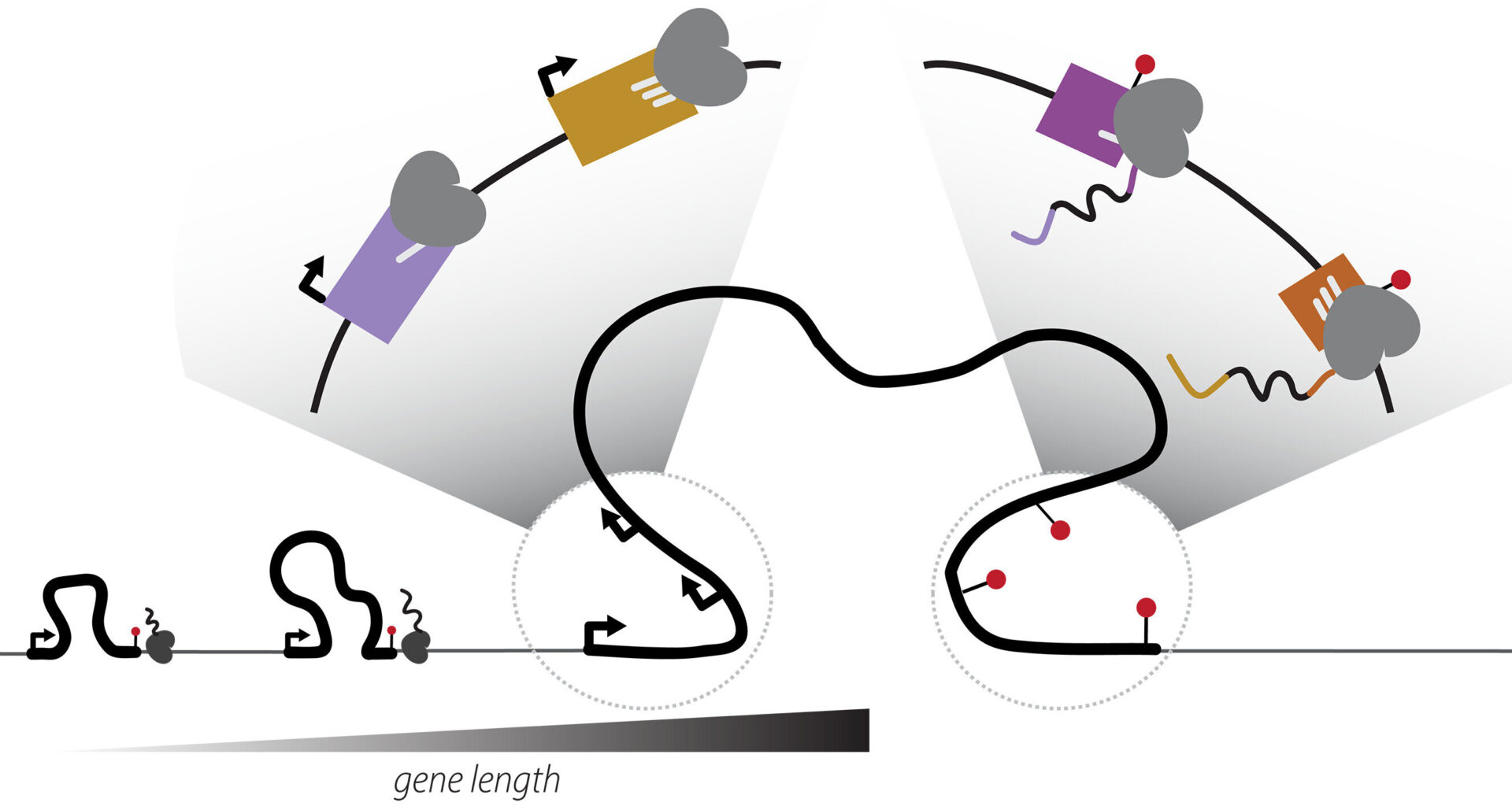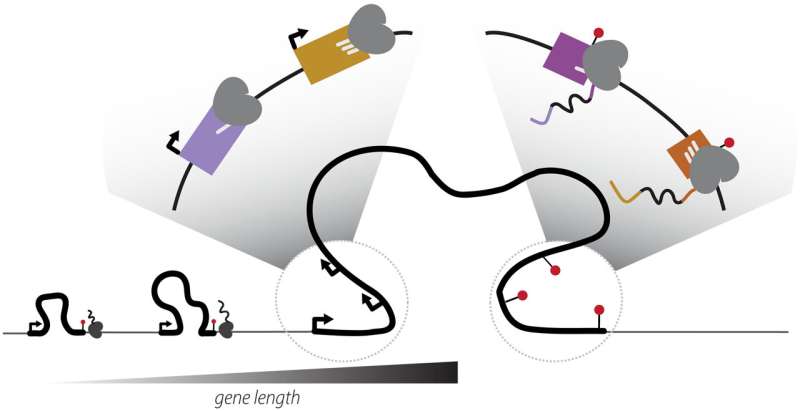
Transcription elongation rates drive the positional coordination of mRNA initiation and termination. Credit: Science (2025). DOI: 10.1126/science.ado8279
Molecular biologists have long believed that the beginning of a gene launched the process of transcription—the process by which a segment of DNA is copied into RNA and then RNA helps make the proteins that cells need to function.
But a new study published in Science by researchers at Boston University and the University of Massachusetts T.H. Chan School of Medicine challenges that understanding, revealing that the beginning and end of genes are not fixed points, but move together—reshaping how cells build proteins and adapt through evolution.
“This work rewrites a textbook idea: the beginning of a gene doesn’t just launch transcription—it helps decide where it stops and what protein you ultimately make,” says Ana Fiszbein, assistant professor of biology and faculty fellow of computing & data sciences, and one of the lead authors of the study.
“For years, we taught that a gene’s ‘start’ only decides where transcription begins. We now show the start also helps set the finish line—gene beginnings control gene endings.”
The discovery offers a promising new strategy for targeting cancer and neurological disorders, as well as developmental delays and aging. When gene transcription is disrupted or misregulated, protein production can become abnormal, potentially causing tumor growth.
The understanding that the beginning and ends of genes are connected could allow physicians to redirect gene expression—restoring healthy protein variants and suppressing harmful ones, without altering the underlying DNA sequence.
“Misplacing a start or an end isn’t a small mistake—it can flip a protein’s domain structure and change its function, too. In cancer, that flip can mean turning a tumor suppressor into an oncogene,” explains Fiszbein.
An oncogene is a mutated gene that has the potential to cause cancer by promoting uncontrolled cell growth and division.
“Our findings show that controlling where a gene begins is a powerful way to control where it ends—and, ultimately, what a cell can do,” she adds.
“We’re not just mapping how genes work—we’re finding new levers to control them. This could become a powerful way to steer cells back toward normal behavior.”
The researchers came to this finding using large-scale genomic data and precise gene-editing experiments involving turning a gene’s start on or off. When they changed where a gene started, it also changed where the gene ended. The same gene could produce hundreds of protein versions—sometimes yielding proteins with different, even opposite, functions.
Christine Carroll, a biology Ph.D. student in Fiszbein’s lab, says the study highlights the power of today’s integrative, data-driven biology—where vast datasets reveal global patterns of gene regulation, and carefully crafted experiments uncover the molecular mechanisms and key variables that bring those patterns to life.
“This adds a new dimension to gene control,” Carroll says. “It’s not just about turning a gene on or off—it’s about determining which version of the gene you get.”
More information:
Ezequiel Calvo-Roitberg et al, mRNA initiation and termination are spatially coordinated, Science (2025). DOI: 10.1126/science.ado8279
Provided by
Boston University
Citation:
Rewriting the rules of genetics: Study reveals gene boundaries are dynamic, not fixed (2025, October 13)
retrieved 13 October 2025
from https://phys.org/news/2025-10-rewriting-genetics-reveals-gene-boundaries.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.